Introduction
The field of thermal analysis comprises methods for the characterization of physical and chemical properties or property changes as a function of temperature. Thermogravimetry allows for the quantifi cation of mass changes, for example, the release of reaction and Reacción de DecomposiciónA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition gases. When these gases are transferred into a gas measuring cell, identifi cation of the released gases becomes also possible. The so-called TGA-FT-IR coupling is a tried-and-tested combination of an analytical and a spectroscopic analysis method.
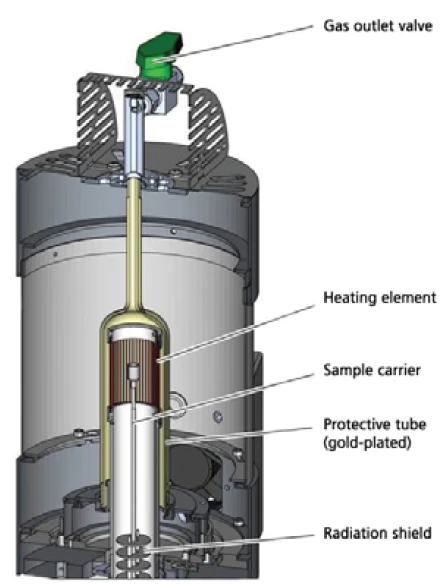
A quite new addition to the established STA 449 F1 Jupiter® (figure 1) is the high-speed furnace (cross-section in figure 2) which can be operated at heating rates of up to 1000 K/min (furnace systems for the most varied of applications covering a temperature range from -150°C to 2400°C are now available).
The influence of the heating rate and the related release rate on the thermogravimetric and spectroscopic measurement results will be discussed in this application note.

Results
a) Polypropylene PP
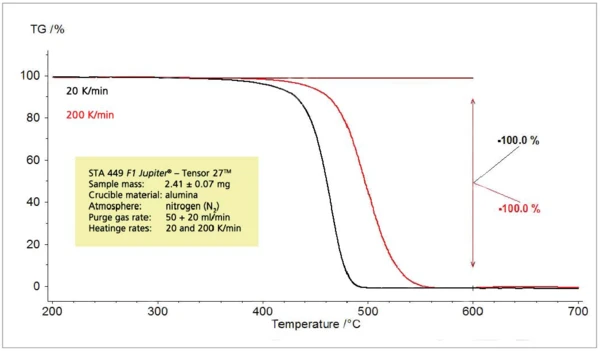
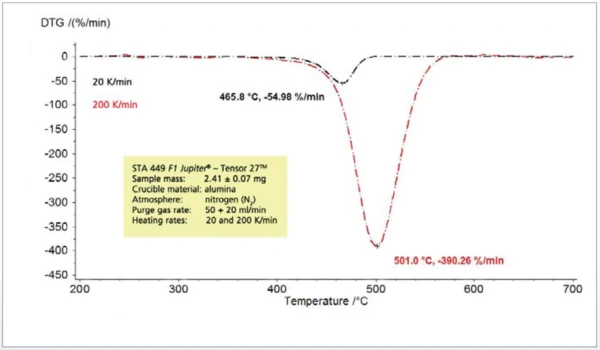
When the heating rate during thermoanalytical experiments is varied, the detected effects are shifted to higher temperatures with increasing heating rates (figure 3). This is well-known and can be used for kinetic evaluations. With an increasing release temperature, the release rate also increases signifi cantly (figure 4). Therefore, the concentration of the sample gases to be analyzed in the constant carrier gas flow also rises and the sample gases can easily be detected and identified. The mass-loss steps, however, are not dependent on the heating rate.
b) CaCO3
The relation between the heating rate and Reacción de DecomposiciónA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition temperature at a constant step height, discussed for the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of propylene, can also be seen during the thermal decomposition of calcium carbonate into calcium oxide and carbon dioxide (figures 5 and 6).
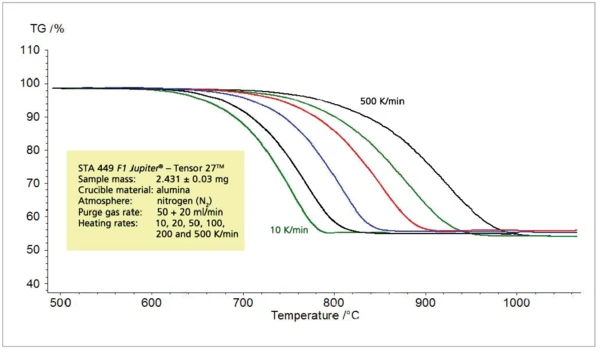
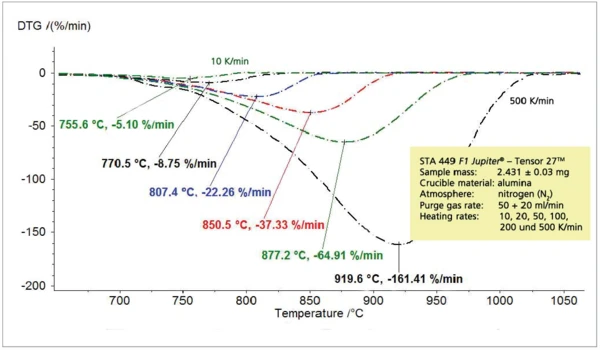
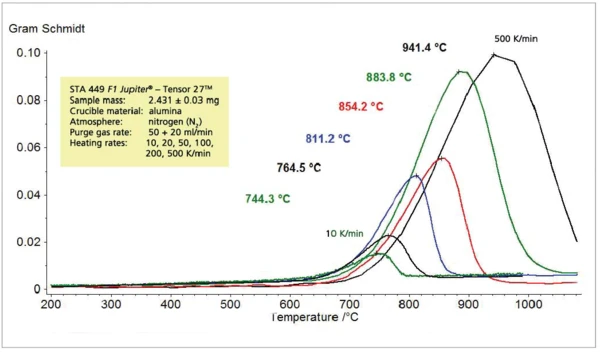
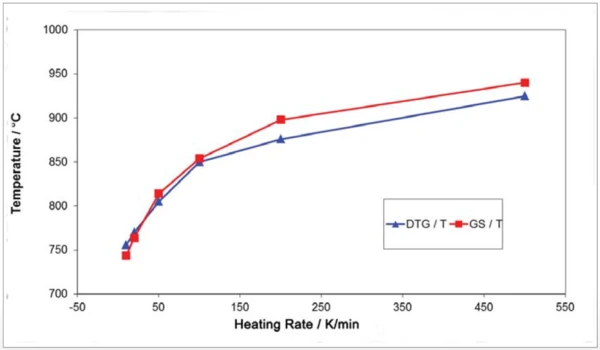
Figure 7 shows the absorption intensity of the corresponding Gram-Schmidt traces which is expected to increase with increasing heating rates. It should be noted here that the transport of the released sample gases into the IR gas measuring cell is hardly delayed due to the fast heating rates. This can be seen by the comparison of the maximum release rate (DTA) with the maximum IR intensity (GS) in figure 8.
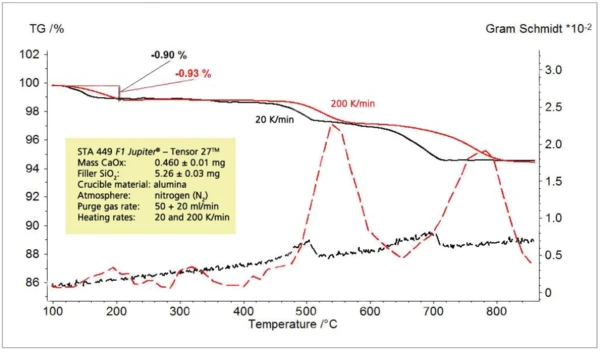
c) CaC2O4 x H2O mixed with SiO2
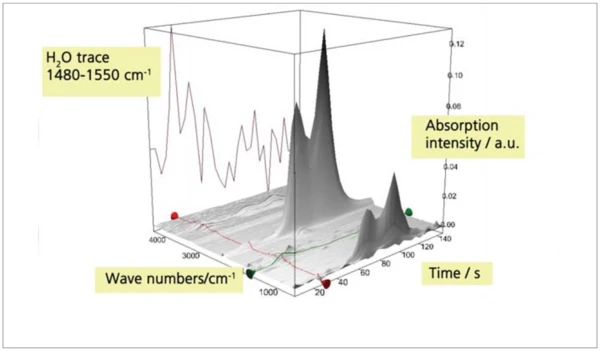
For investigation of the detection limit, a mixture of calcium oxalate monohydrate (CaC2O4 x H2O) and quartz sand (SiO2) was prepared. The selected mixing ratio was 1:10 so that the expected release of water corresponded to approximately 1% of the sample mass. The thermal release of approximately 1% of water from this mixture could not be detected at a heating rate of 20 K/min; employing a heating rate of 200 K/min, however, it could clearly be detected (figure 9 to 11).
Summary
By means of fast heating rates of up to 500 K/min, it is possible to considerably increase the release rates of gasesous products from a sample. Therefore, their concentration also increases in comparison with the carrier gas, resulting in a signifi cant improvement of the detection limit of a TGA-FT-IR coupling.