Measuring degradation related changes in molecular weight and rheology during polycaprolactone processing
Introduction
Polycaprolactone (PCL) is a synthetic polymer that has recently received increasing attention thanks to its biodegradability. Its most common use is in the manufacture of polyurethanes or as a plasticizer for other polymers such as PVC. It is also often used in molding and prototyping thanks to its low Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature and is used as a feedstock in some additive manufacturing (3D printing) systems. Finally, it is also used in some drug delivery applications as a control release mechanism, in the same way as polylactic acid (PLA) or polylacticco-glycolic acid (PLGA). A potential advantage over PLA and PLGA is that PCL has a slower degradation rate and therefore may allow for slower drug release.
As with all polymers, PCL’s molecular properties (e.g., molecular weight) willstrongly affect its bulk properties such as strength, toughness, and melt-flow. Being biodegradable, PCL is at a high risk of degradation during processes such as extrusion for molding, particularly at high temperatures. Some mechanisms have been described in the literature to reduce this risk. For instance, extrusion in the presence of carbon dioxide (CO2) can reduce the melt flow viscosity of PCL by acting as a ‘molecular lubricant’. Decreasing the viscosity of the polymer reduces the temperature at which extrusion can be performed and could thereby protect the polymer from degradation during the process [1].
In this application note, a commercially available sample of PCL was extruded alone and in the presence of CO2. Rotational rheometry was used to study the polymer’s melt viscosity, while Malvern GPC measurements were made of the virgin sample before and after extrusion.
Methods
The PCL sample was extruded using a Rondol bench top extruder at screw speeds of 30 rpm through a 1 mm slit die both in the presence (150 °C) and absence (160°C) of CO2 [1].
The melt viscosities of the samples were also measured on a Kinexus Ultra+ rotational rheometer using an active hood peltier plate cartridge at 150°C and parallel plates with a diameter of 20 mm and a measuring gap of 1 mm. A frequency sweep was performed to determine the sample’s complex viscosity. The measurement was conducted under a purge of nitrogen to reduce the risk of oxidative degradation occurring.
The three samples were measured by multi-detector GPC on a Malvern OMNISEC system including refractive index (RI), UV-Vis, light scattering (right-angle light scattering (RALS) and low-angle light scattering (LALS)), and viscometer (IV) detectors. The samples were dissolved to concentrations of approximately 3 mg/ml and separated over two Malvern T6000M mixed bed SVB columns.
Test Results
Figure 1 shows a chromatogram of the virgin PCL sample. As can be seen, the sample is well resolved and signal-to-noise is good across all detectors. The chromatogram has been overlaid with measured molecular weight and intrinsic viscosities.
Figure 2 shows overlays of the RI, RALS and viscometer detectors for the virgin, extruded and extruded with CO2 samples. The chromatograms show triplicate measurements of each sample overlaid. Small differences are visible on the different detectors. Although the differences appear small, the repeatability of the measurements is excellent.
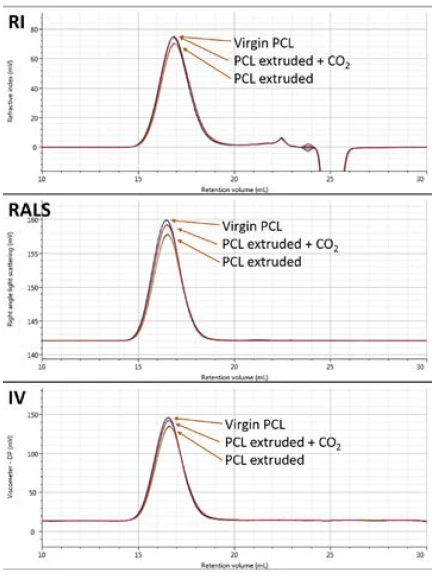
Table 1 shows the calculated numerical results for these samples. The virgin PCL has an average measured molecular weight of 114.6 KDa. After extrusion, this has dropped to 103.8 KDa; however, when CO2 was injected directly into the barrel of the extruder, it allowed the extrusion to take place at a temperature 10°C lower. The net effect of the use of CO2 and lower extrusion temperature was to mitigate the degradation of the polymer by around 40% and maintain the molecular weight at 108.1 KDa. A similar, though less well defined trend is observed in other measured parameters such as the intrinsic viscosity and hydrodynamic radius for the samples.
The samples were then measured on a rotational rheometer to see how their bulk properties (melt viscosity) was affected by these molecular changes. Melt viscosity is typically strongly dependent on a sample’s molecular weight. The same trend appears to be present in the rotational rheology data.
Table 1: Measured results for the three PLC samples by means of the multi-detector SEC
Virgin PLC | PLC extruded | PLC extruded + CO2 | ||||
|---|---|---|---|---|---|---|
Measurement | Mean | % RSD | Mean | % RSD | Mean | % RSD |
| RV (mL) | 16.84 | 0.01142 | 16.9 | 0.08211 | 16.87 | 0.04973 |
| Mn (g/mol) | 73,660 | 0.7468 | 66,380 | 1.656 | 69,420 | 0.5563 |
| Mw (g/mol) | 114,600 | 0.1184 | 103,800 | 0.1682 | 103,11 | 0.1908 |
| Mw/Mn | 1.556 | 0.6447 | 1.564 | 1.656 | 1.557 | 0.4961 |
| IVw (dL/g) | 1.244 | 0.1226 | 1.183 | 0.01061 | 1.186 | 0.5057 |
| Rh(ŋ)w (nm) | 12.7 | 0.06067 | 12.6 | 0.0539 | 12.24 | 0.2383 |
| M-H a | 0.6797 | 1.494 | 0.6806 | 2.391 | 0.694 | 1.775 |
| M-H log K (dL/g) | -3.327 | -1.552 | -3.323 | -2.454 | -3.402 | -1.871 |
| Recovery (%) | 98.44 | 0.03634 | 94.54 | 0.08072 | 97.3 | 0.2655 |
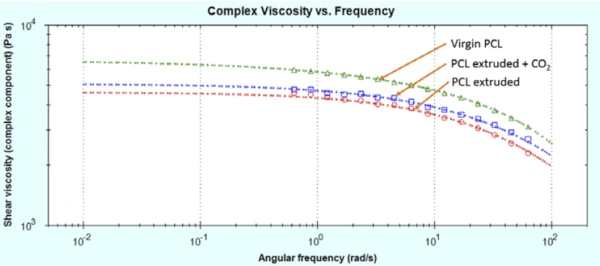
As can be seen in figure 3, the virgin PCL has the highest melt viscosity. The sample extruded in the absence of CO2 has a lower melt viscosity. This is partially mitigated by extruding the sample in the presence of CO2 at a lower extrusion temperature.
Finally, the multi-detector GPC data was studied to see if there were any changes in the PCL structure as a result of the extrusion. The Mark-Houwink plot shows intrinsic viscosity as a function of molecular weight and can therefore be used to assess changes in molecular structure and conformation. It is most Adiabatic temperature riseThe adiabatic temperature rise (delta Tad) is the observed temperature rise (delta Tobs) of a self-heating decomposition reaction under adiabatic conditions in consideration of the PHI-factor.commonly used in the study of polymer branching.
At first glance at the Mark-Houwink plot for the PCL samples, it appears that they overlay well and there are no changes in polymer structure. However, upon closer inspection, it appears that the sample extruded in the absence of any CO2 (i.e., the most degraded) has also undergone a small change in structure. Figure 4 shows the overlay of triplicate measurements demonstrating the repeatability of this extremely small but clear difference.
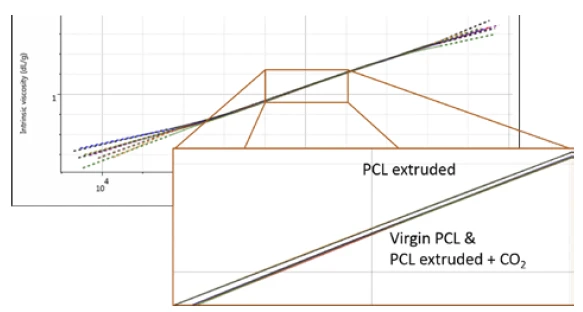
This change could be due to a degradation of sample branching, however, this sample was believed to be linear. It could also potentially be related to small differences caused by some hydration of the polymer which was not dried out before the experiments. Nevertheless, this finding provides an interesting avenue for further potential investigations.
Conclusions
The results demonstrated in this application note demonstrate how the processing conditions can affect both the underlying and bulk properties of a polymer such as PCL. Here, the molecular weight and melt viscosity of a PCL sample was seen to drop when the sample was extruded in the absence of CO2 at 150°C. However, the effect of this was partially mitigated by the inclusion of CO2 during the extrusion process. By interacting with some of the molecules within the sample, CO2 effectively acts as a 4‘molecular lubricant’ to reduce the viscosity of the sample. In doing so, it means that PCL can be extruded at a lower temperature, and this, in turn, protects the polymer from some of the observed degradation.
This difference was successfully observed at the molecular level using multi-detector GPC, and at the bulk level using rotational rheometry. In this way, both technologies can be used to correlate changes at the molecular level with those observed in the final product.
The reduced melt viscosity that comes from a lower molecular weight is likely to affect any mold produced with this sample. It is also likely to affect Crystallinity / Degree of CrystallinityCrystallinity refers to the degree of structural order of a solid. In a crystal, the arrangement of atoms or molecules is consistent and repetitive. Many materials such as glass ceramics and some polymers can be prepared in such a way as to produce a mixture of crystalline and amorphous regions.crystallinity and mechanical properties, and subsequently, in the case of drug delivery applications, would influence drug release timings. Any products created from it are therefore more likely to have wider performance tolerances and greater variation. On the other hand, by extruding with CO2 this effect was mitigated and using this procedure would likely protect the product’s performance.
The use of multiple technologies to characterize the polymer allows for a clear measurement and understanding of the underlying changes that are occurring to the polymer during extrusion and processing. By understanding and controlling these changes through strategies such as extruding with CO2, manufacturers can maintain higher product quality and tighter control on product quality, reducing failures and increasing product value.