
13.08.2020 von Dr. Gabriele Kaiser
The Role of DSC in Lyophilisation Processes
Many APIs (active pharmaceutical ingredients) or formulations, especially those based on biopharmaceuticals, are thermally sensitive and unstable in aqueous solutions. Particularly the latter property is highly unfavorable if they are to be administered as an injectable dosage form like vaccines. In order to achieve higher stability and longer shelf life, such drug ingredients must be dried. However, due to their thermolability, it is not feasible to remove the water just by heating. Freeze-drying or lyophilisation is a gentle alternative to transform APIs and/or mixtures between APIs and excipients into usable and storable forms without thermal treatment.
Learn how thermal analysis can help determine the parameters of the freeze-drying process.
The Freeze-Drying Process Consists of Three Steps
The freezing phase, the so-called ´primary drying` and, finally, the ´secondary drying`.
- The freezing phase during which the substance is frozen at a selected freezing rate
- The so-called ´primary drying`: The ice will be removed from the freeze-concentrated solution by sublimation under reduced pressure. At this stage, the product temperature is usually at about -35°C to -20°C.
- The ´secondary drying`: The temperature is further increased to dry the product to the final moisture level via desorption of the water contained in the matrix. In order to receive a stable cake, a water concentration of e.g., 1% or less is required [1].
To avoid any activity loss of the drug substance, cryo- or lyo-protectants such as sugars (e.g., sucrose or trehalose) or polymers are typically added.
The Collapse Temperature is Decisive
A critical parameter for the setup of a lyophilisation process is the collapse temperature, often called Tc. At this temperature, the material either melts or softens so that it can no longer support its own structure and begins to flow. For this reason, the substance should be kept below Tc within the ´primary drying` phase. However, a too low process temperature leads to an unacceptable slow progression. Therefore, it is important to know the value of the critical temperature. For crystalline systems, the maximally tolerable temperature corresponds to the eutectic melting temperature [1 – 3]. Only below that point is the system completely solid. But most freeze-dried formulations contain amorphous phases and in this case, the collapse temperature is close to the glass transition temperature of the maximally freeze concentrated solute (Tg´). In many cases, the Tc is slightly higher than the Tg´ while the exact difference between both temperatures is formulation-dependent [2].
DSC (Differential Scanning Calorimetry) Determines the Tg´ …
DSC instruments are designed to detect changes in the specific heat capacity of materials as it takes place while passing a glass transition. Fig. 1 shows the DSC signal during heating of a frozen 10% sucrose solution. Freezing as well as heating were performed with the instrument at 5 K/min. The evaluated glass transition temperature of the maximally concentrated solution (Tg´, given here as midpoint) results to -32°C and fits well to literature values [4]. The height of the endothermal step is expressed as Δcp and amounts here to 0.28 J/(gK).
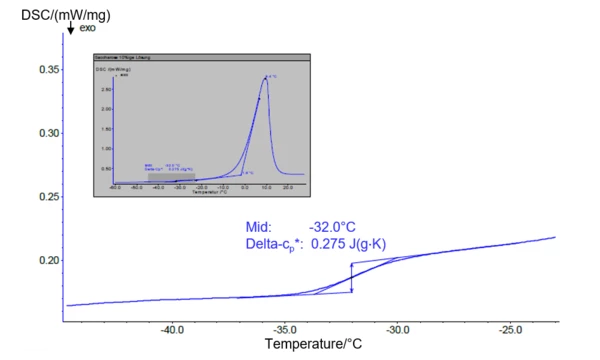
As mentioned above, the Tg’ is slightly below the collapse temperature in many formulations and therefore represents a rather conservative upper limit, although this has no influence on the quality of the final product. If the glass transition step is overlapped by RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation effects or crystallization peaks related to one of the formulation components, temperature-modulated DSC (TM-DSC or mt-DSC) can help for separation.
… and the Glass Transition Temperature of the Dried Product
Many lyophilized products stay in its amorphous form after drying. As water shows a softener effect, the glass transition temperature of the amorphous phase is directly related to the entrapped residual water content. Thus, DSC can furthermore be used to determine the drying status of the material. Literature: [1] E. Meister and H. Gieseler, A significant comparison between collapse and glass transition temperatures, European Pharmaceutical Review, online, September 2008 https://www.europeanpharmaceuticalreview.com/article/1479/a-significant-comparison-between-collapse-and-glass-transition-temperatures/ [2] V. Kett, Development of Freeze-dried Formulations Using Thermal Analysis and Microscopy, American Pharmaceutical Review, online, September 2010 https://www.americanpharmaceuticalreview.com/Featured-Articles/36885-Development-of-Freeze-dried-Formulations-Using-Thermal-Analysis-and-Microscopy/ [3] H. Schiffter-Weinle, Immer schön trocken bleiben, Deutsche Apothekerzeitung, online https://www.deutsche-apotheker-zeitung.de/daz-az/2016/daz-44-2016/immer-schoen-trocken-bleiben [4] F. Franks, Freeze-drying of bioproducts: putting principles into practice, European Journal of Pharmaceutics and Biopharmaceutics, 1998, 45, p 221–229