
Introduction
Epoxy resins are materials that have been widely used in a variety of applications, including the coating and coloring of bicycle lanes or crossroads, the surface-coating of floors in parking garages and warehouses, and electronics. Nowadays, epoxy resins are also used as light-weight materials for the rotor blades of windmills in order to produce electricity from renewable sources. The rotor blades of windmills are an excellent example demonstrating the need to have precise knowledge about the progress of Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing in order to prevent failures in production. A single rotor blade of 60 meters in length has a mass of approximately 15 tons – which would also be the amount of waste in the case of an unsuccessful Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing approach. This example clearly demonstrates why knowledge about the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing reaction and its kinetics is of great importance in optimizing the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing process with respect to temperature, time and efficiency.
The Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing reaction of epoxy resins can be studied with different techniques within the family of thermal analysis methods. The production of heat during the curing reaction can be detected with differential scanning calorimetry (DSC) [1]. Laser Flash Analysis (LFA) can be used to detect changes in thermophysical properties such as Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity [2]. Flammersheim and Opfermann demonstrated how to use the specialized NETZSCH Thermokinetics [3] software in order to study the timeand temperature-dependent progress of curing reactions [4]. Viscosity changes can be investigated by means of dielectric analysis (DEA) [5-11] or dynamic-mechanical analysis (DMA) [12]. Pretschuh et al. correlated the two techniques in order to study the curing of aminoplast resins [13].
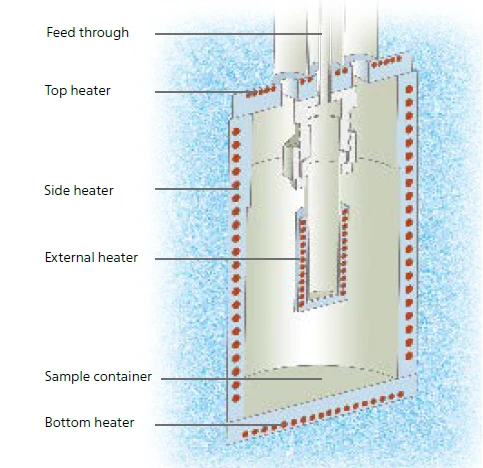
This work introduces the use of an additional caloric measurement technique. The NETZSCH Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).Multiple Module Calorimeter (MMC) 274 Nexus® (figure 1) offers three different measurement modules. The Accelerating Rate Calorimetry (ARC)The method describing isothermal and adiabatic test procedures used to detect thermally exothermic decomposition reactions.ARC® Module can be used for thermal hazard studies; the Coin-Cell Module is specialized for the investigation of batteries; and the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module can be used to evaluate caloric data from a single heating run. In contrast to the widely used and well-known technique of differential scanning calorimetry (DSC), the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module of the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC can handle samples up to a volume of 2 ml. For heating the samples, there are two options available: either a constant heating rate or a constant level of power. By using information about both the power supplied to the sample and the heating rate, a heat-flow signal can be calculated.

Using metals such as indium, tin and bismuth, both the temperature and the sensitivity of the instrument can be determined. At 1000 to 9000 mg (sample volume about 1 ml), the typical sample masses are considerably higher for the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC than sample masses used for DSC, which are typically between 5 and 10 mg. Even so, the evaluated uncertainty for the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module of the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC is about 1% for temperature determinations and less than 5% for enthalpy determinations.
This work points out similarities and differences in sample preparation, measurement modes and results obtained for the curing reaction of epoxy using the NETZSCH DSC 214 Polyma versus the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.scanning module of the MMC.
Sample Preparation and Measurement Conditions
In order to prevent the epoxy resin sample from beginning to slowly react already during storage, it is placed in a refrigerator at -20°C. Prior to sample preparation, the storage container is removed from the refrigerator and warmed at ambient temperature for approximately one hour. The sample now has a honey-like viscosity and is taken with a spatula and dropped into the vessel or crucible for MMC and DSC measurements, respectively. After sample preparation, the storage container is placed back into the refrigerator. A comparison of the measurement conditions for the two instruments is shown in table 1.
In order to study the curing of epoxy resins with the MMC, the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module with an external heater is used (figure 3). The external heater is placed directly around the sample vessel and supplies constant power to the sample; in this case, 1000 mW. Due to the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity and mass of the vessel along with the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity and mass of the sample, the heating rate will not be exactly constant. The ratio of masses and specific heat capacities is known as the Φ-factor (or Thermal inertiaThe thermal inertia is equivalent to the PHI-factor. Both describe the ratio of mass and specific heat capacity of a sample or sample mixture compared to that of the vessel or sample container.thermal inertia). According to ASTM E1981 [14], it can be expressed in the following equation:
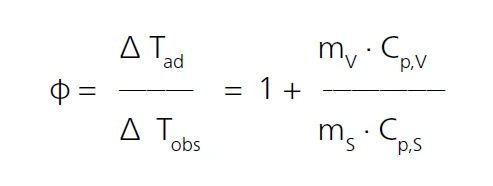
T: temperature
ad: AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios
and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic
obs: observed
m: mass
V: vessel
cp: Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity
S: sample
Tab 3: Measurement conditions
| DSC 214 Polyma | MMC 274 Nexus® |
|---|---|---|
| Vessel material | Aluminum | Stainless steel |
| Vessel type | Concavus® crucibles, pierced lid | Closed |
| Vessel mass | 51.478 mg | 7230.84 mg / 6914.95 mg |
| Heating | 5 K/min | Constant power (1000 mW) |
| Atmosphere | Nitrogen | Air |
| Purges gas rate | 40 ml/min | Static |
| Temperature range | RT ... 290°C | RT ... 290°C |
| Sample mass | 12.553 mg | 1096.50 mg / 1178.00 mg |
Ultimately, the resulting heating rate will be influenced by the thermal behavior of the sample itself. Since the curing of epoxy resins is an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic reaction, the heat of reaction will increase the heating rate temporarily. Heat losses to the surrounding environment are suppressed by the guard heaters positioned on the sides, top and bottom of the calorimeter. These heaters will track the sample temperature independently from the constant power mode of the external heater. A schematic drawing of the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module of the MMC 274 Nexus® is shown in figure 3.
Results and Discussion
Aproximately 1000 mg of the epoxy resin sample is heated via the external heater of the MMC 274 Nexus® using a constant power level of 1000 mW. The power input leads to a temperature increase rate of about 4.5 K/min up to 150°C. With the beginning of the curing reaction of the epoxy resin, the heat of reaction increases the heating rate up to a maximum of 14.0 or 14.5 K/min, respectively. Due to the additional input of power from the reaction enthalpy, the measured sample temperature increases much faster during the ongoing curing process. Figure 4 depicts the results of a repeat measurement of the epoxy resin curing carried out with the MMC 274 Nexus®.
Besides the sample temperature (solid lines) and heating rate (dashed lines), the MMC also allows for measurement of the sample pressure (dashed-dotted lines) since the feed-through on top of the sample vessel is connected to a pressure gauge. The pressure inside the closed vessel system increases continuously with temperature and begins to increase more quickly after curing due to the start of Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the cured product.
The sample heat flow can be calculated using the constant power signal of the external heater and the resulting heating rate of the sample.
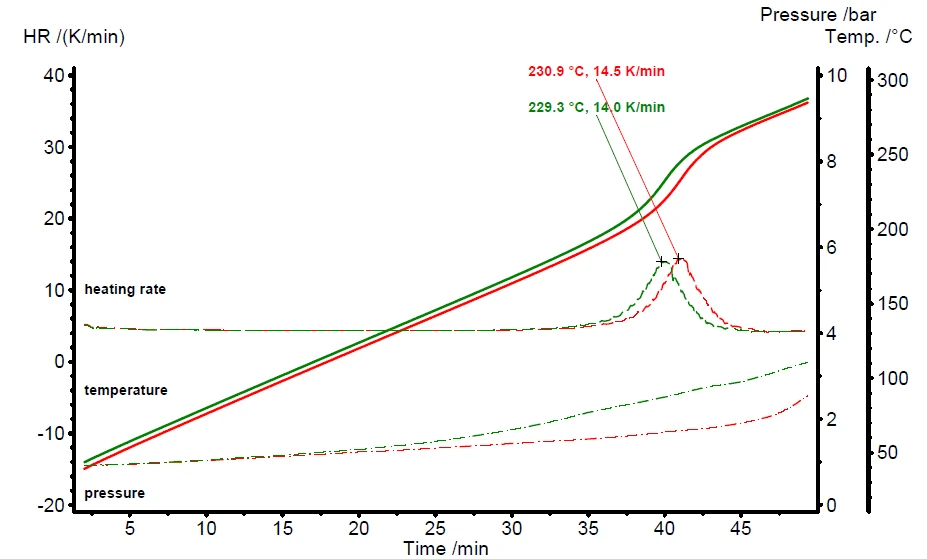
Figure 5 depicts the results of a repeat measurement including the heat-flow signal of the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic curing reaction. Conducting a measurement along the same lines using the DSC 214 Polyma yields comparable results even though both the measurement modes and the sample masses are significantly different. Figure 6 compares the results of the measurement using the DSC 214 Polyma with those from the MMC 274 Nexus®.
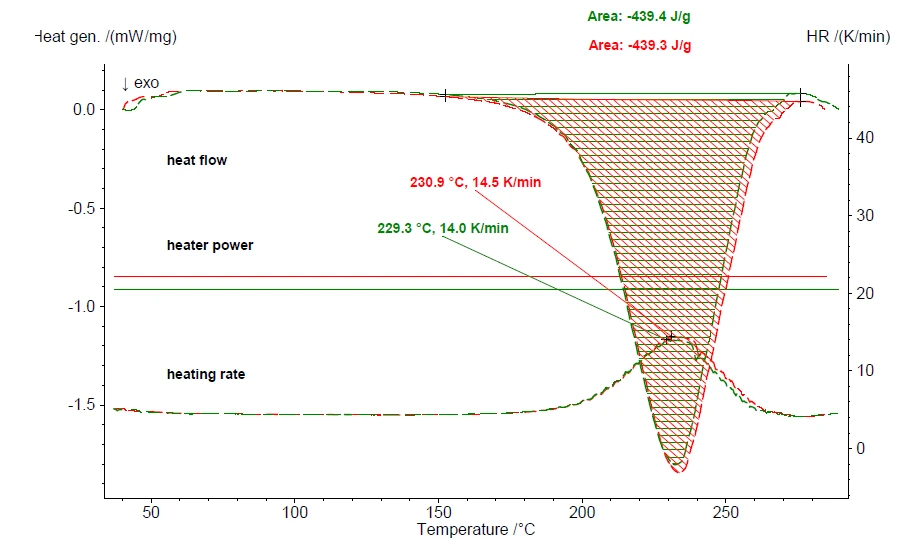
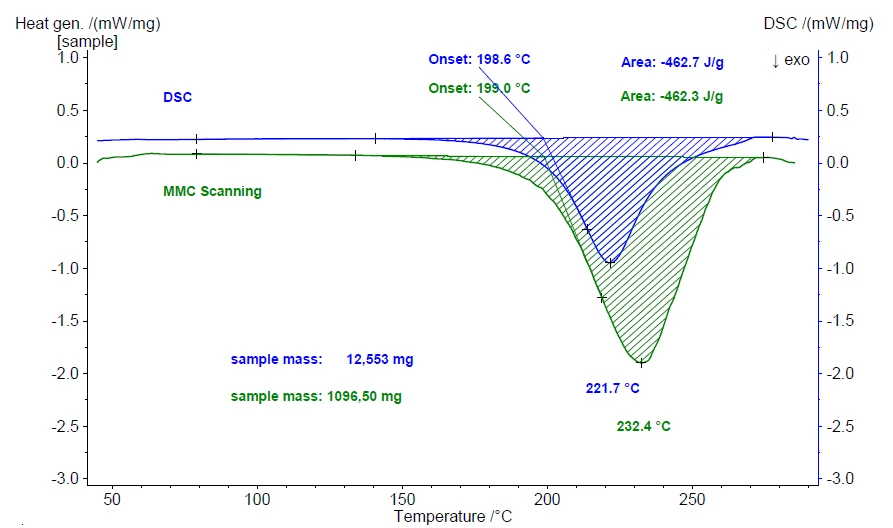
The evaluated values for both the enthalpy of curing and the extrapolated onset – representing the beginning of the curing reaction – are identical for the two techniques within the uncertainty limits. The maximum peak temperature, however, differs by more than 10 K. This significant difference is due to the enormous difference in sample mass: 12.553 mg (DSC) versus 1096.50 mg (MMC). It simply takes more time to complete the reaction when the sample mass is more than 80 times higher.
Taking into consideration that the results for both the DSC and MMS techniques are scales with the identical range of heat flow (DSC right scale, MMC left scale), the visual impression of the peak areas is different. However, the evaluated values for the extrapolated onset and reaction enthalpy are identical within the uncertainty limits. This seems to be inconsistent, but indeed, it is not. Temperature-scaled results of dynamic heating or cooling treatments include the heating rate. From DSC experiments, we expect the heating rate to be constant (here 5 K/min). For the MMC, a constant power input was used – therefore, the heating rate depends on the sample behavior. As can be seen from figure 5, the heat of reaction during the MMC measurement more than triples the measured heating rate at the sample from 4.5 K/min prior to the reaction to 14.5 K/min during the curing reaction. This increase in heating rate let the peak area for the MMC results appear much larger compared to the DSC results at a constant rate of 5 K/min. Since enthalpy evaluation takes the heating rate into account, the evaluated values are almost identical although the visual impression of the peak areas is different.
Conclusion
The curing reaction of epoxy resins can be investigated with various measurement techniques. Depending on which property change is being studied, methods such as DMA, DEA or LFA may be applied. DSC is certainly the most widely used technique for investigating curing reactions due to the strong ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic heat of reaction. This work shows that in addition to differential scanning calorimetry, another caloric technique can also serve for the investigation of a curing reaction. In contrast to DSC, the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.scanning module of the NETZSCH Multiple Module Calorimeter MMC 274 Nexus® can study samples on the gram scale and yields comparable results.