Introduction
In the following, some terms specific to the pharmaceutical field are explained:
- Thermal stability
- Compatibility
- PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).Polymorphism
- Pseudo-PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism
1. Thermal Stability
Standard ASTM E2550 describes the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability of a material as the “temperature at which the material starts to decompose or react and the extent of mass change using thermogravimetry”. It adds that “the absence of reaction or Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition is used as an indication of the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability”.
Figure 1 displays the TGA curve of acetylsalicylic acid during heating to 600°C in a nitrogen atmosphere.
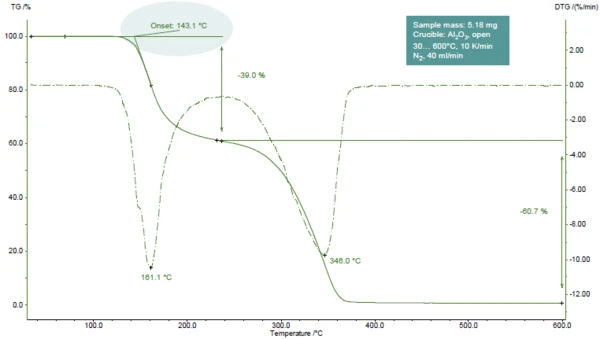
Two mass-loss steps are detected, easily recognizable by the two peaks in the DTG curve (1st derivative of the TGA curve). TGA-FT-IR investigations showed that during the first step, acetic acid (main part) and salicylic acid evolve. During the second step, salicylic acid and CO2 (resulting from the further Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of acetylsalicylic acid) are released [1].
Each of these mass-loss steps is determined by:
- the temperature
- the mass change
Theoretically, three temperatures can be displayed for a mass-loss step:
- Peak temperature of the DTG (1st derivative of the TGA curve)
- Extrapolated onset temperature according to the standard ISO 11358-1. This is “the point of intersection of the baseline at the beginning of the measurement and the tangent to the TGA curve at the point of maximum gradient”
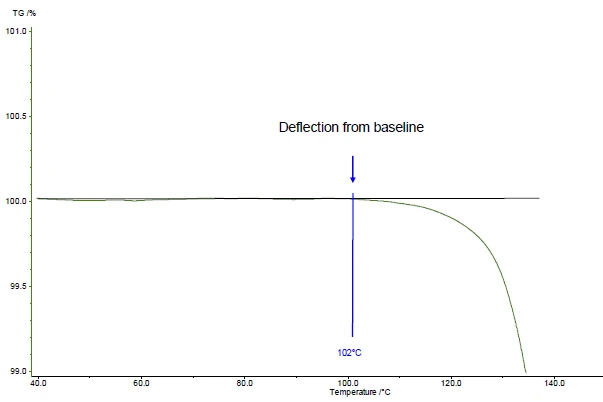
Onset temperature according to ASTM E2550. This is the “point in the TGA curve where a deflection is first observed from the established baseline prior to the thermal event”
In the presented example, the first mass-loss step occurs at 161°C (peak of the DTG curve, figure 1), at 143°C (extrapolated onset temperature of the TGA curve, figure 1) or 102°C (onset temperature according to ASTM E2550, figure 2). This third value is used for evaluation of the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability.
The method is limited to materials that react or decompose in the investigated temperature range, and cannot be used for a sublimation or VaporizationThe Vaporization of an element or compound is a phase transition from the liquid phase to vapor. There exists two types of vaporization: evaporation and boiling.vaporization.
Notes regarding the measurement conditions:
As the results are affected by the sample mass, the atmosphere (gas and flow rate), the heating rate and the crucible type, it is crucial to mention the measurement conditions. For the same reason, the results for two samples can only be compared if the measurements are carried out under exactly the same conditions.
In general, the following measurement conditions are recommended:
- Sample mass: between 1 and 10 mg, for example, 5 mg
- Heating rate: 10 to 20 K/min (lower for energetic reactions: 1 to 10 K/min)
- Flow rate of the atmosphere: 20 to 100 ml/min
In the presented example, the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability at 102°C for acetylsalicylic acid is given for a measurement in a dynamic nitrogen atmosphere (gas flow: 40 ml/min) carried out on a 5 mg-sample at a heating rate of 10 K/min (figure 2).
Analysis by Kinetics Neo
A thermogravimetric measurement shows the effect of the temperature on a material in a specified atmosphere. If the observed mass loss is dependent on the heating rate, then it is possible to use TGA measurements at different heating rates to carry out a kinetic analysis of the reaction. For this, NETZSCH offers the Kinetics Neo software. It allows for modelling the kinetics of single- to multi-step reactions. This software can assign each individual step to different reaction types with kinetic parameters of its own, such as activation energy, order of reaction, and pre-exponential factor. Based on the results, Kinetics Neo is able to simulate the reaction(s) for user-specified temperature programs, for example for long-time isotherms. That’s why the predictions calculated with Kinetics Neo yield information about the shelf life with regard to the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability of a material, i.e., the amount of time that it remains stable under a specified atmosphere and temperature conditions.
An example of determination of the shelf life with regard to the thermal stability of a pharmaceutical product is explained in the NETZSCH Application Note 122 [2].
Notes regarding determination of the shelf life of a drug with regard to thermal stability:
- Carry out TGA measurements at different heating rates
- Carry out the kinetics evaluation with Kinetics Neo
- Use the kinetics model determined to predict the sample behavior for specified temperatures and times
- Validate the kinetic model by comparing a measurement at an IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature with the curve calculated by Kinetics Neo.
Important Remarks:
- Factors other than temperature and atmosphere also influence the shelf life of a product, e.g., humidity, light or loss of miscibility in the case of ointments. That’s why the predictions performed with TGA and Kinetics Neo don’t yield information about the complete shelf life of a product, but only about its shelf life with regard to thermal stability.
- The predictions are valid for substances that are in the same physical state at the temperature of prediction and at the temperature of the beginning of Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition. If a material is in a solid state at room temperature and melts before it begins to decompose, then the kinetic analysis of the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition is valid only for the liquid state. In such case, no prediction using the calculated model can be carried out at temperatures below the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point.
2. Compatibility
In general, a pharmaceutical formulation contains one active pharmaceutical ingredient and several excipients.
The active pharmaceutical ingredient, also called API (Active Pharmaceutical Ingredient), is the substance which has a “direct effect on the diagnosis, cure, mitigation, treatment or prevention of disease” [3].
There are different goals for the various excipients: they can facilitate the manufacturing process, improve the appearance of the final product (color, taste) and help the API to be delivered correctly.
The presence of the excipients in the formulation should not affect the efficacy, stability or safety of the drug. In other words, it should be ensured that the API and excipients are compatible.
Initial information about the compatibility of a drug and excipient can be obtained with thermal analysis, more specifically with DSC and TGA.
Notes regarding the determination of interactions on API and excipient:
- Run DSC and TGA measurements on API and, separately, on excipient
- Mix API and excipient (50/50 weight)
- Run DSC and TGA measurement on the mixture of API+excipient
DSC Curves of API, Excipient and Mixtures
Figure 3 displays how DSC curves yield information about a potential interaction between two components. A resulting DSC curve that shows no interaction between API and excipient (figure 3c) indicates that the excipient is recommended for the formulation using the API. The occurrence of a new peak in the mixture, the disappearance of a peak, or a change in the melting peak (in shape, position, or enthalpy) would indicate that there is an interaction between the two components (figure 3d). However, this doesn’t necessarily mean that the drug and excipient are not compatible. Additional investigations would have to be carried out with other techniques (X-ray, spectroscopy, chromatography, etc.) to confirm incompatibility.
a) DSC curve of API with melting peak

b) DSC curve of excipient with melting peak
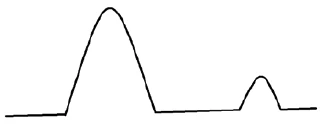
c) DSC curve of the mixture API+excipient WITHOUT interaction between the two components. One melting peak is detected at the same temperature as in the DSC curves for the individual components. This means API and excipient are compatible.
c) DSC curve of the mixture API+excipient WITHOUT interaction between the two components. One melting peak is detected at the same temperature as in the DSC curves for the individual components. This means API and excipient are compatible.
The Superposition feature in the evaluation software by NETZSCH allows for depiction of the curve that would be obtained for a mixture if no interaction between the two components occurs. To carry this out, the curves of the individual substances are loaded in the evaluation software, and the “superimposed” curve is calculated. It is then very easy to make a comparison between the measured curve of the mixture and the curve calculated by means of Superposition.
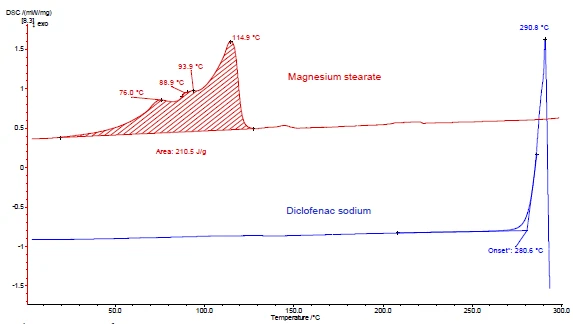
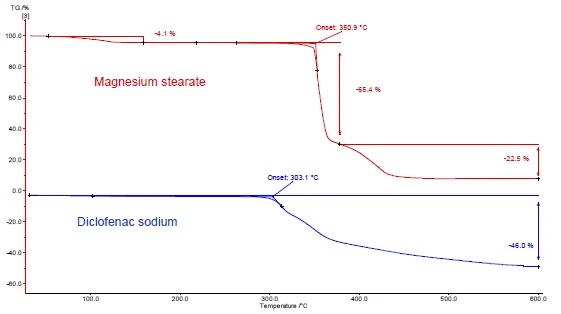
Figures 4 and 5 show how to proceed with the example of diclofenac sodium and magnesium stearate. DSC and TGA measurements were carried out. Figures 4a and 5a display the DSC and TGA curves, respectively, of the two substances during heating.
The endothermal peak between room temperature and 130°C detected in the DSC curve of magnesium stearate (figure 4a, red curve, top) is due in part to the evaporation of water. It corresponds to a mass loss in the TGA curve (4.1%) for this temperature range. The water release peak is overlapped by the melting of magnesium stearate [9].
Diclofenac sodium (figure 4a, blue curve, below) shows an endothermal peak at 291°C, corresponding to its melting. An ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic process immediately following the melting is associated with a mass loss of 46% and results from the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of diclofenac.

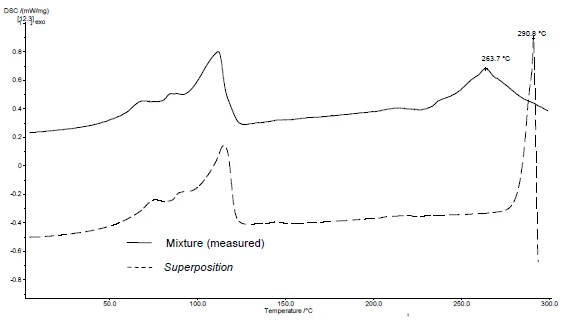

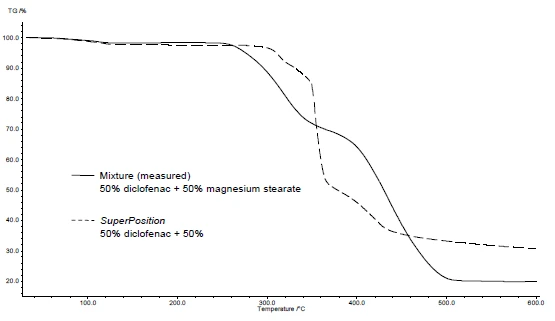
The application of SuperPosition (figures 4b, 5b) allows for a comparison of the measured curve of the mixture with the calculated curve that would be obtained in the case of no interaction. No difference between the two curves would indicate a compatible mixture.
In this example, decomposition of the mixture begins at 278°C, i.e., at a lower temperature than for the excipient alone (figure 5c). The melting peak typical for diclofenac is no longer exhibited in the mixture. Instead, a broad endothermal peak at 264°C is detected (figure 4c).
The fact that there are differences detected in the example indicates that there is an interaction between diclofenac sodium and magnesium stearate (figures 4c and 5c).
A further example of a compatibility study on diclofenac sodium with different excipients by means of DSC and TGA is given in the NETZSCH Application Note 120 [4].
3. Polymorphism
PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).Polymorphism is the ability of a material to exist in more than one crystal form. The different polymorphic forms of a pharmaceutical substance are usually called α, β, … or I, II, ... or A, B, ..., where the modification α/I/A is the most stable one.
In the pharmaceutical industry, PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism is very challenging because even though two polymorphic substances have the same chemical composition, they differ in their properties. Because a polymorphic substance can change its structure over time, unexpected changes in its bio-availability, physical properties, stability, etc. can occur during storage. For this reason, as well as concerning patent registration, it is crucial to be aware of, and knowledgeable about, the existence of all of the potential modifications of a polymorphic substance and the properties, stability, and quality of each.
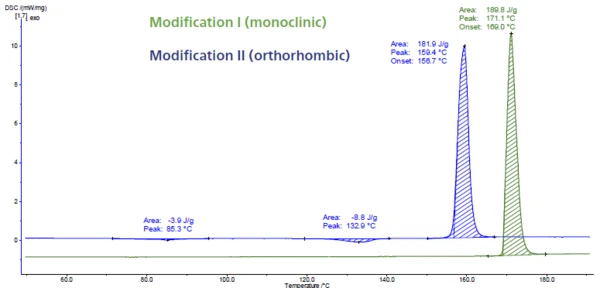
Figure 6 displays the DSC measurement on paracetamol. This API (Active Pharmaceutical Ingredient has three modifications called I, II and III. Modification III is unstable and thus difficult to characterize. Modifications I and II differ in their thermodynamic stabilities and their compression abilities. They can be easily identified by means of DSC because the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature is detected at different temperatures. The melting peak at 169°C (extrapolated onset temperature, green curve) is typical for the monoclinic form. It is the modification with the highest Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point and also the most stable one. The peak at 157°C (extrapolated onset temperature, blue curve) belongs to the orthorhombic form that features better compression properties [5, 6].
Although form II could be directly compressed without the addition of an excipient to improve compressibility, commercial paracetamol is manufactured from the monoclinic form (form I) because of its better stability [7, 8].
Other examples of the characterization of different modifications of a polymorphic substance are given in the NETZSCH Application Note 127 [10].
4. Pseudo-Polymorphism
Two pseudo-polymorphic modifications have different crystal forms resulting from hydration or solvation.
In a solvate, the solvent molecules are entrapped in the crystalline structure of the substance. If this contains more than two solvents, it is referred to as a hetero-solvate.
In a hydrate, the solvent in association with the drug is water.
The characterization of solvates and hydrates is carried out with thermogravimetry, possibly coupled with evolved gas analysis. A TGA measurement yields information about the amount of solvent/water present in a sample, and thus about the degree of solvation/hydration. Coupling allows for the identification of solvents released during heating.
Conclusion
By means of thermal analysis, particularly DSC and TGA, the different properties of API and excipients can be investigated. This, in turn, allows for the determination of the thermal stability, compatibility and PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism and pseudo-PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism of pharmaceuticals.