28.06.2022 by Prof. Dr. Ing. Sascha Englich
Epoxy Resins – Reactive Polymers as a Basis for Injection-Moldable Compounds
Material analysis is of great importance for component as well as mold and process design in the automotive industry. Read, how differential scanning calorimetry contribute to the optimization of epoxy resin injection molding and learn in our second blogarticle of this new blog series even more about injection-moldable compounds.
Prof. Dr. Ing. Sascha Englich is a professor of plastics engineering at the Steinbeis University of Berlin. As part of a new blog series on the optimization of epoxy resin injection molding using differential scanning calorimetry, he explains today, amongst others, the difference between uncured and cross-linked material state and talks about simulation models.
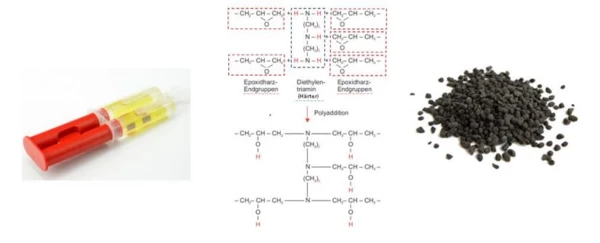
Epoxy resins are not as unfamiliar to us as they first appear. After all, anyone who has ever repaired anything with a 2-component adhesive is already familiar with this material and its particular features. Thereby, a resin is mixed with a hardener (figure 7, left), thus setting a chemical cross-linking reaction (figure 4, center) – i.e., the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing process – in motion. Components generally designated as “carbon fiber” or “carbon” are also based on systems such as these epoxy resin-hardeners. In this case, they, which here also serve as an adhesive, initially infiltrating the fiber bundles during production and forming a firm bond. However, the same chemical principle of resin and hardener can be found in thermoset molding compounds for injection molding (figure 7 on the right), already described in our first article by May 11th, “Thermoset Injection Molding in E-Mobility”. Here, too, a resin-hardener system was discussed, but adjusted such that it occurred as a solid and exhibited almost no chemical reaction at moderate temperatures (slightly cooled to room temperature). Therefore, these materials can be manufactured as ready-compounded molding materials (resin, hardener, filler and reinforcing materials, additives, etc.) in a granulate form and stored for a certain time. Only at elevated temperatures does the chemical cross-linking reaction set in at an accelerated rate, which can be taken advantage of in the processing of heated injection molds.
This material setup, initially consisting of a resin and hardener which then combine to form a 3-dimensional network, also offers the ability to investigate the material structure and its changes by means of thermoanalytical methods (e.g., DSC).
Uncured Versus Cross-Linked Material State
Here, a differentiation must be made between the uncured and the cross-linked material state. The uncured, oligomer resin is present in the amorphous state so that the phase transformation from solid to liquid (Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition) can be measured by means of DSC analysis (Differential Scanning Calorimetry). In the signal of the heat flow, a “step” occurs (in figure 2, approximately between 60°C and 90°C). The reason for this is the material’s change in Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity during phase transformation. Evaluation of the step describes the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition range with Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature TG_0 (figure 2, Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition) of the uncured resin, thus giving an initial indication of the lower processing temperature necessary for plasticizing in the injection molding machine.
Looking at the further progression of the heat-flow signal, an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effect occurs at higher temperatures, represented as a peak (figure 2, complex peak [ISO]). This ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peak characterizes the chemical cross-linking reaction with the peak area representing the heat of reaction and the integral of the reaction enthalpy. The course of the integral (figure 2, evaluation routine using peak integral) describes the cross-linking process. If the peak integral is derived as a function of time (da/dt), the reaction dynamics are obtained. From the processing point of view, for example, an upper temperature limit for plasticizing can be derived from the starting point of the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peak, and an optimal tool temperature can be derived from the integral’s peak.
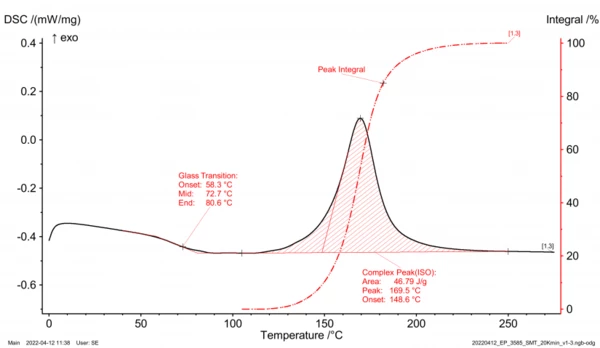
Figure 3 depicts the different results of the DSC analysis for an epoxy resin in various cross-linking states. As already described before, the uncured starting material (Figure 3, upper graph) shows a clear Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition range with Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature of TG_0 as well as the subsequent ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal cross-linking peak. The integral of the peak (area) describes the total cross-linking enthalpy.
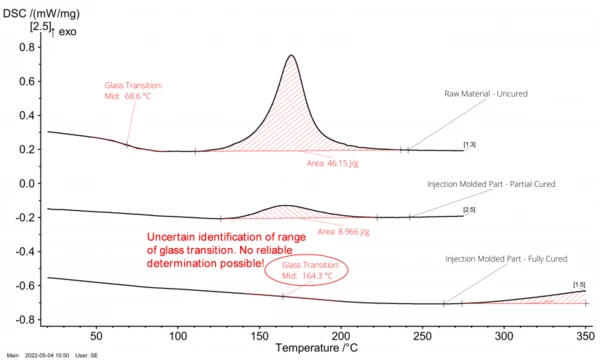
The graph in the middle of figure 3 shows the DSC signal of an injection-molded component, but with incomplete cross-linking. A Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition range can no longer be recognized since it continues to increase dynamically during the measurement with the post-CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization starting at elevated temperatures. As already indicated, the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peak describes post-cross-linking or residual cross-linking. From the ratio of the entire cross-linking enthalpy and the residual cross-linking enthalpy, the degree of cross-linking can be determined:
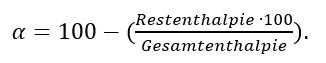
In In the example shown, the component’s Degree of CureThe degree of curing describes the conversion achieved during crosslinking reactions (curing). degree of cure amounts to around 81%; i.e., the injection molding process should be optimized again in this case.
The lowest graph in figure 3 depicts the DSC signal of an entirely cross-linked component. Since no further chemical cross-linking reaction occurs, there is also no ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effect to observe. In theory, the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition at complete Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing, TG_1, can be determined instead. However, in the case of injection molding compounds which are typically very highly filled, this is so little pronounced that the determination is not always reliable, especially since the evaluation range for TG_1 often overlaps with the range of thermal degradation. This becomes visible as an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal curve increase at very high temperatures (shaded area beginning at approx. 270°C). Therefore, determination of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature of cured components by means of DSC is not recommended. Thermomechanical analysis (TMA) or Dynamic Mechanical Analysis (DMA) would be much better solutions to this end.
Reaction Dynamics for Simulation
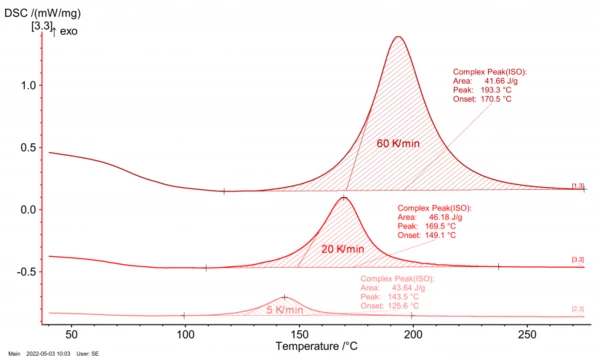
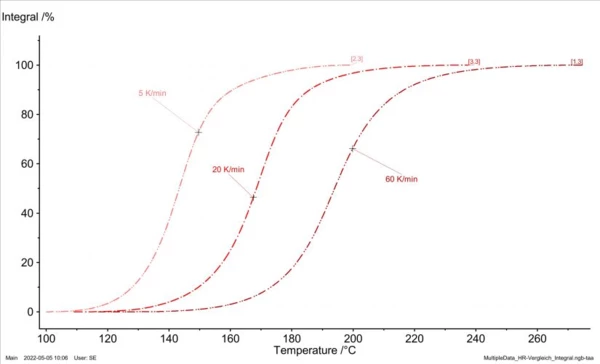
Besides using DSC analysis for testing the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing state, it also serves as a basis for generating material data for process and Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing simulations. To this end, several DSC analyses are carried out at different heating rates (figure 4 and figure 5) and then the courses of the reaction dynamics are transferred into mathematical models. For injection molding of epoxy compounds, for example, the so-called Kamal-Sourour model is widely used:
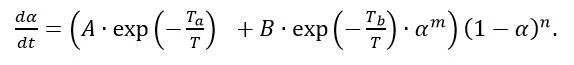
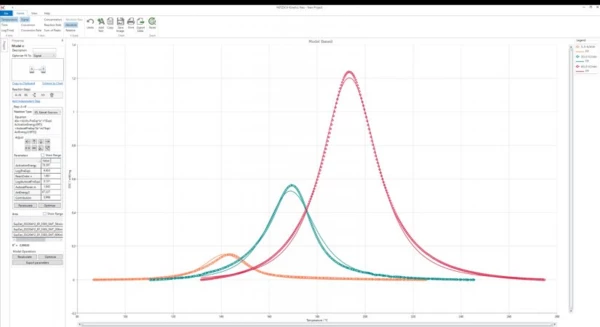
In simulation, such models now allow for computation of any cross-linking scenarios as a function of time and temperature. Data fitting can be performed, for example, by means of NETZSCH Kinetics Neo as shown in figure 6.
With regard to sample preparation for epoxy resin-based injection molding compounds, the following procedure has become established: Aluminum crucibles/lids are used, with the lids being pierced. (For other types of molding compounds such as those based on phenolic resins, special pressure-tight crucibles should be used.) The granules are ground to a powder, if possible without thermal signature, and “carefully” pressed into the crucible (figure 14). This significantly increases contact with the crucible bottom as well as the thermal conduction within the sample, leading to consistent and reproducible DSC curves.

Learn more about the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing optimization using DSC in the next blog article of our new blog series by Dr. Sascha Englich.
For more information in advance, go to NETZSCH Analyzing & Testing.