
04.12.2023 by Dr. Gabriele Kaiser
IQ, OQ – Instrument Qualification in the Pharmaceutical Field and What It Means for Thermal Analysis Users
This article is dedicated in particular for users of Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), based on USP <1058>.
In the pharmaceutical sector, precision and adherence to process regulations are of utmost importance. Amongst others, the United States Pharmacopeia (USP) provides a guideline, also for carrying out installation qualification (IQ) and operational qualification (OQ). They form the foundation for ensuring accuracy and reliability in pharmaceutical research and quality control.
IQ and OQ are part of the so-called analytical instrument qualification (AIQ) process at the user´s lab to confirm that an instrument is properly installed, works correctly and yields the expected results. A key term in this context is “demonstration of fitness for purpose”. In the following, we would like to explain in more detail what’s behind that, mainly on the basis of USP <1058>, the corresponding chapter in the US pharmacopeia.
Risk-Based Approach
Depending on the complexity of the instrument or on how critical the measurement is, the instruments are classified into three groups: A, B and C. However, depending on the purpose for which the device is used, the same instrument type can belong to one group or the other.
- Group A includes standard equipment without measurement capability or user requirement for calibration. If they are obviously working well, no further qualification will be necessary.
- Group B includes instruments providing measurement values or experimental conditions, which can affect a measurement and therefore need routine calibration, maintenance or performance checks. Such instruments may have firmware but no software, which is updated by the user.
- Group C includes instruments with a significant degree of computerization and complexity. They are connected to a computer and generate electronic records. For such instruments, all elements of qualification (see below) plus software validation are required. However, software validation and instrument qualification can be combined into a single activity because the software is generally essential for running the instrument.
In USP <1058>, only a limited number of instrument examples are given: pH meters and ovens for group B as well as mass spectrometers and HPLC instruments for group C. In older USP versions, both (DSC) and (TGA) are explicitly classified as group C.
The Four Qualification Phases
These are DQ (Design Qualification), IQ (Installation Qualification), OQ (Operational Qualification) and PQ (Performance Qualification). PQ is also sometimes called “user acceptance test (UAT)”. For complex systems, this framework can be extended by function specifications (FS) and/or factory acceptance tests (FAT), if necessary. Most important is that all required activities be performed in logical order. In addition, all qualification activities should be predefined and contemporaneously documented.
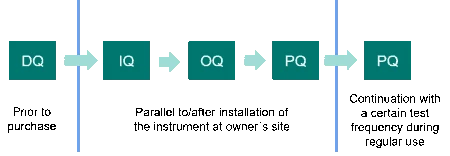
Design Qualification
DQ takes place prior to the purchase decision and defines the requirement profile of the DSC or TGA device. This includes the functional and operational specifications as well as the intended purpose of the instrument and, finally, shows that the selected instrument is appropriate. DQ can be performed by the instrument manufacturer or by the user. Some laboratories additionally issue a separate user requirement specification (URS) list, which prepends the DQ. In the URS, future users specify what they want the instrument to be capable of doing.
Installation Qualification
IQ stands for the documented evidence that the particular DSC or TGA instrument, including software, accessories, etc. is delivered as designed and as specified, that the planned environment is appropriate and that the instrument is properly installed. It applies to new or pre-owned DSC or TGA instruments and takes place after installation and commissioning of the instrument at the owner’s site.
Operational Qualification
OQ follows the installation qualification. It is a documented collection of activities with the objective of verifying that the DSC or TGA instrument works properly in the selected environment and fulfills the operational specifications given in the Design Qualification (DQ) and/or the user requirement specifications (URS).
Performance Qualification
PQ is the last of the four qualification phases and documents continuous suitability of the DSC or TGA instrument for its intended use under real operating conditions. This usually also implies using typical on-site applications and the laboratory’s own testing substances. The test frequency depends, amongst other aspects, on the ruggedness of the DSC or TGA device, the experience of the operator and how critical the analytical method is. Another part of performance qualification is preventive maintenance and documentation of repairs and changes in equipment. Preventive maintenance also comprises periodic calibration of the DSC or TGA instrument.
Responsibility
The ultimate responsibility for qualification of the DSC or TGA instrument lies with the user, whereas the term ‘user’ covers not only the operator but also that person’s supervisors, the associated instrument experts and the organization’s management. Instrument manufacturers and suppliers can only advise and assist.
What NETZSCH Offers
NETZSCH Analyzing & Testing offers various services to support users during the qualification process of NETZSCH DSC or TGA units. These are:
- IQ-OQ documents (in standard version and – optionally – customized), including a template for recurring PQ tests
- Installations with subsequent processing of the IQ-OQ-(PQ) documents
- Maintenance contracts comprising calibration of the instruments on a regular basis
Interested? Please contact your local specialist:
Watch also our video about the Development and Quality Control of Pharmaceuticals with Thermal Analysis and Rheology
Pharma Application Book Available!
The book contains eight chapters on more than 260 pages about:
- Thermal analysis methods (DSC, TGA, STA and Gas Analysis)
- Characterization of amorphous and crystalline phases
- Purity
- Thermal stability
- Oxidative stability
- Storage conditions and shelf life
- Polymorphism and Compatibility











