Introduction
Polyamides are semi-crystalline polymers characterized by good mechanical resistance; this allows them to be used for various technical applications, such as cable protection in the automotive industry and robotics. Polyamide powder is also a popular material for SLS (Selective Laser SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering), a 3-D printing method allowing for creating objects of any shape.
However, polyamides are also very sensitive to water. The molecular chains of polyamides contain polar amid groups that attract polar liquids such as water, so that this polymer absorbs the moisture present in the environment. The water molecules increase the free volume in the polyamide chain gaps, which results in polymer swelling and easier sliding of the molecular chains at mechanical load. This leads to lowering of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition and is called water-induced plasticizing effect. [1, 2, 3]
Consequently, the water uptake drastically affects the mechanical, thermal and electrical properties of polyamides. In particular, increasing the water content leads to a decrease in stiffness and strength, while toughness increases. [3, 4, 5]
DSC Investigates the Influence of Humidity on the Glass Transition of Polyamide
In the following, the influence of humidity on the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of polyamide 6 (PA6) is investigated. To this end, DSC measurements were performed on samples containing different levels of water content between 0% and 4.9%.
Table 1 summarizes the measurement conditions. The Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of PA6 is usually overlapped with the endothermal peak due to water evaporation. This predestines it to perform temperature-modulated DSC measurements that separate the reversing (e.g., Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition) from the non-reversing effects (e.g., evaporation of volatiles, Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing) [6].
Table 1: Measurement Conditions
| Device | DSC 300 Caliris®, H-Module | |||
| Sample | Dried (0-% humidity) | 1.2-% humidity | 3.3-% humidity | 4.9-% humidity |
| Sample mass | 9.92 mg | 10.04 mg | 10.26 mg | 10.44 mg |
| Crucible | Concavus® (aluminum) with pierced lid | |||
| Temperature range | -60°C to 240°C | |||
| Heating rate | 5 K/min | |||
| Period | 60 s | |||
| Amplitude | 0.8 K | |||
Glass Transition Temperature of PA6
Figure 1 depicts the total heat flow of the sample with 1.2% humidity, which corresponds to a conventional DSC curve without modulation. The endothermal step at 38.8°C (midpoint) indicates the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of polyamide 6. However, this evaluation is not accurate because the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition is overlapped with an endothermal peak, most probably due to the starting water release contained in the sample and from RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation effects. Before melting takes place at 224.2°C (peak temperature), the amorphous part of the PA6 crystallizes partly, explaining the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peak at 193.3°C (peak temperature) in the DSC curve.

Figure 2 displays the total heat flow along with the raw DSC signal obtained during the temperature-modulated measurement. The total heat flow (continuous line) is equivalent to a standard DSC measurement, as described above. The raw signal (dashed line) shows how the material actually responds to temperature modulation.

In figure 3, the total heat flow is separated into a reversing and a non-reversing part. This allows for separation of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition and the evaporation peak. The Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition is detected in the reversing part of the DSC signal and the evaporation effect in the non-reversing part.
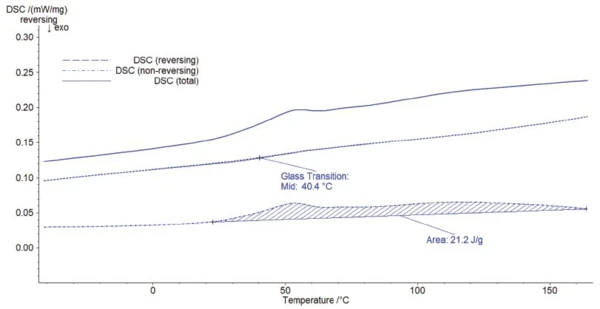
Subsequently, the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition is evaluated accurately (midpoint at 40.4°C). The non-reversing signal, however, highlights that the endothermal peak is much wider than initially assumed. This effect due to RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation and evaporation is related to an enthalpy of 21.2 J/g.
Influence of Humidity on the Glass Transition Temperature of PA6
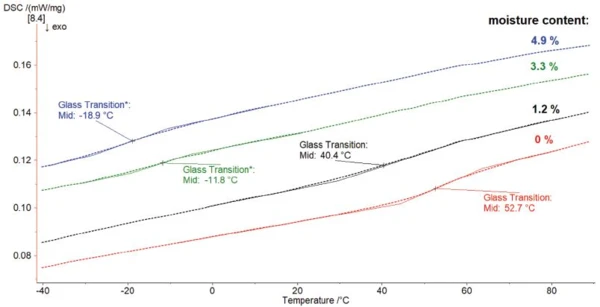
Figure 4 shows the reversing signal of the different samples. The higher the moisture content, the lower the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature. There is more than 70°C difference between the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of the dry sample and the PA6 containing 4.9% water.
Conclusion
Due to their hygroscopic nature, polyamides absorb humidity from their environment. This, in turn, influences the properties and hence processing of the material. Even a small amount of water in PA6 will drastically decrease its glass transition. For this reason, the moisture content of the sample is an essential parameter to check and control.
A reliable and fast way to proceed is to perform temperature-modulated DSC measurements with the DSC 300 Caliris®.