Introduction
In order to investigate the effect of particle size on the physical properties of crystalline materials, different particle sizes of crystalline substances that were generated by grinding were analyzed by thermal analysis methods, such as thermogravimetry (TGA) [1], differential scanning calorimetry (DSC) [2] and dilatometry [3].
Relatively small variations in particle size produced significant changes in thermal processes that were investigated by these methods.
The thermal processes that were investigated can be divided into four categories:
- Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting of metals (solid-liquid)
- Reactions at the particle surface (combustion of carbon)
- Release of gaseous reaction products (dehydration and Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition)
- SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering
Melting
Particle size variation in the milli- and micrometer range, according to Schmid [4], does not significantly influence the melting behavior of particles. For spherical particles with diameters greater than 50 nm, particles at the surface account for less than 6% of the bulk and therefore have negligible effect. For smaller particle sizes (r < 25 nm), the percentage of coordinatively unsaturated particles near the surface in-creases, causing a signifi cant decrease in the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature [6] according to the Reifenberger model [5].
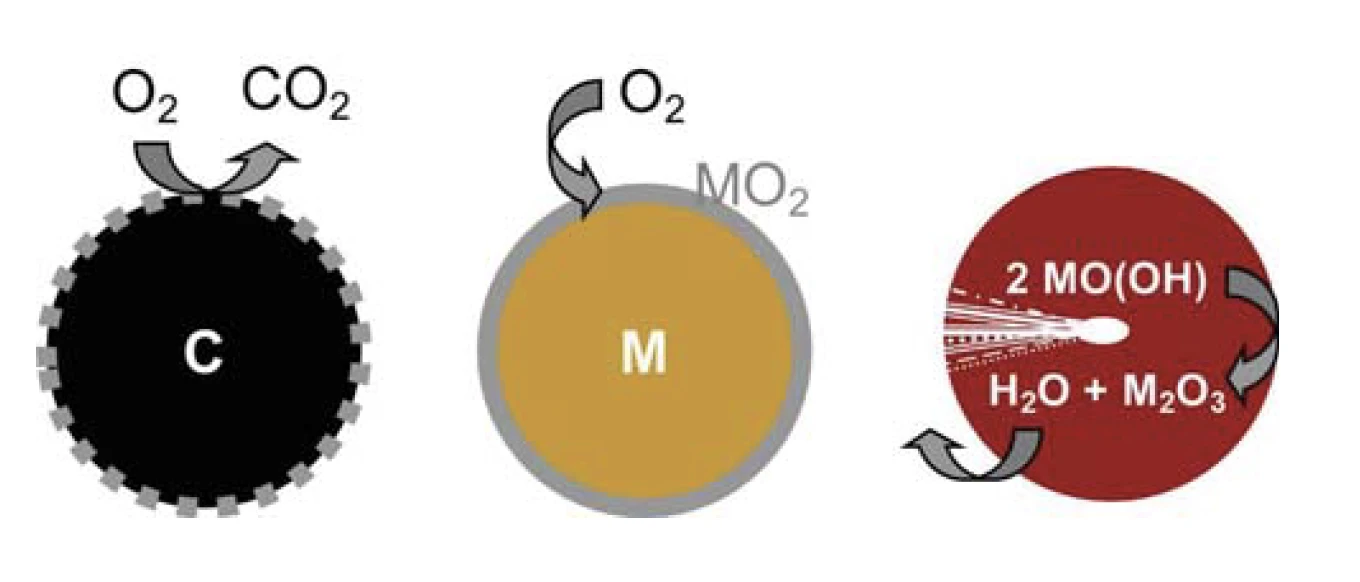
Reactions at the Particle Surface
The combustion of carbon particles can be used as a model for a surface reaction. The gaseous oxygen can be supplied evenly to the particle surface and reacts there to form CO2, a gaseous and therefore easily removable product. A fresh, reactive surface is generated by the reaction itself. The carbon particle decreases in size until it is completely converted to CO2. By contrast, the metal oxide surface layer produced during the OxidationOxidation can describe different processes in the context of thermal analysis.oxidation of metal particles presents a passive barrier layer that hinders oxygen access to the metallic core beyond a certain thickness, thereby preventing quantitative conversion (figure 1).
Measurement Results
Despite their comparable particle sizes (~50 nm), different types of carbon black exhibited very different combustion behavior, as shown in figure 2. The differences are likely due to differences in the porosities of the materials, which affect their surface areas. Thus, the particle size alone is only a rough determination of OxidationOxidation can describe different processes in the context of thermal analysis.oxidation behavior.
Release of Gaseous Reaction Products
Although Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reactions require no additional gaseous reactant, they are, nevertheless, significantly influenced by transport processes. Although the surface area is not crucial in this case, the distance over which released gases must be transported from the interior to the surface of the particle via pores or channels depends on particle size. Hence, this process is considerably more efficient for very small particles.
The CaCO3 (figure 3) and goethite (figure 4) examples illustrate the effect of smaller particle size in lowering the temperatures at which the materials decomposed with the release of CO2 or H2O [6]. The thermogravimetric results confi rm that the stoichiometries of the gases released are unaffected by variation of the particle size.
Thermokinetic analysis of the dehydration of α-FeOOH (goethite) to α-Fe2O3 (hematite) showed that the formal kinetic model for the reaction was simpler for the small particles than for large particles. Measurements at different heating rates were modeled by a reaction process consisting of two consecutive nth order steps and an activation energy of 150 kJ/mol [7]. Quantification of the massloss steps between 120°C and 350°C confirms the expected values for the stoichiometric conversion of goethite to hematite. The rate of mass loss (DTG) – indicated with dashed lines – shows that the reaction peak is shifted to lower temperatures with smaller particle sizes. The photo in figure 4 shows the change in the appearance of the goethite samples with varying particle size.
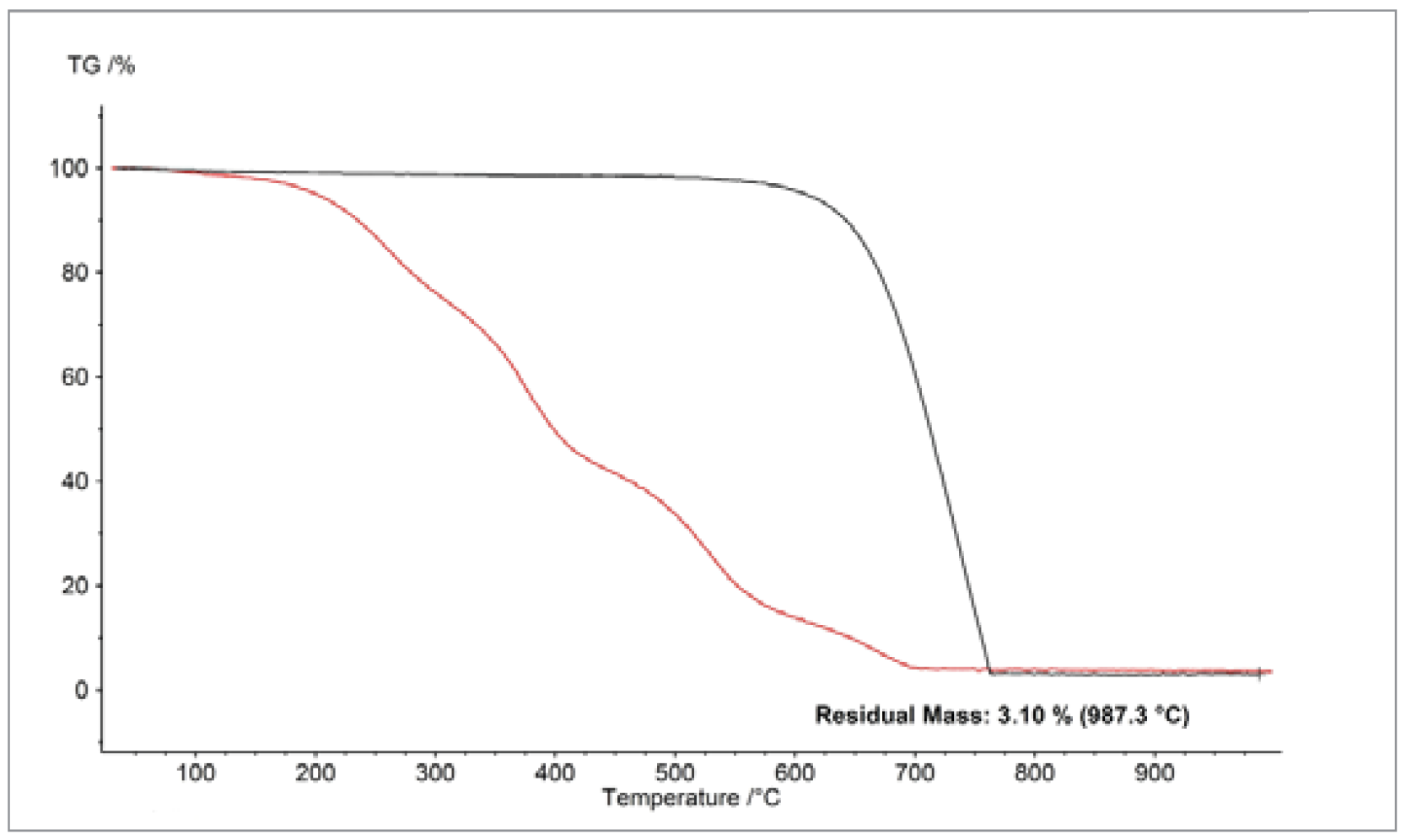
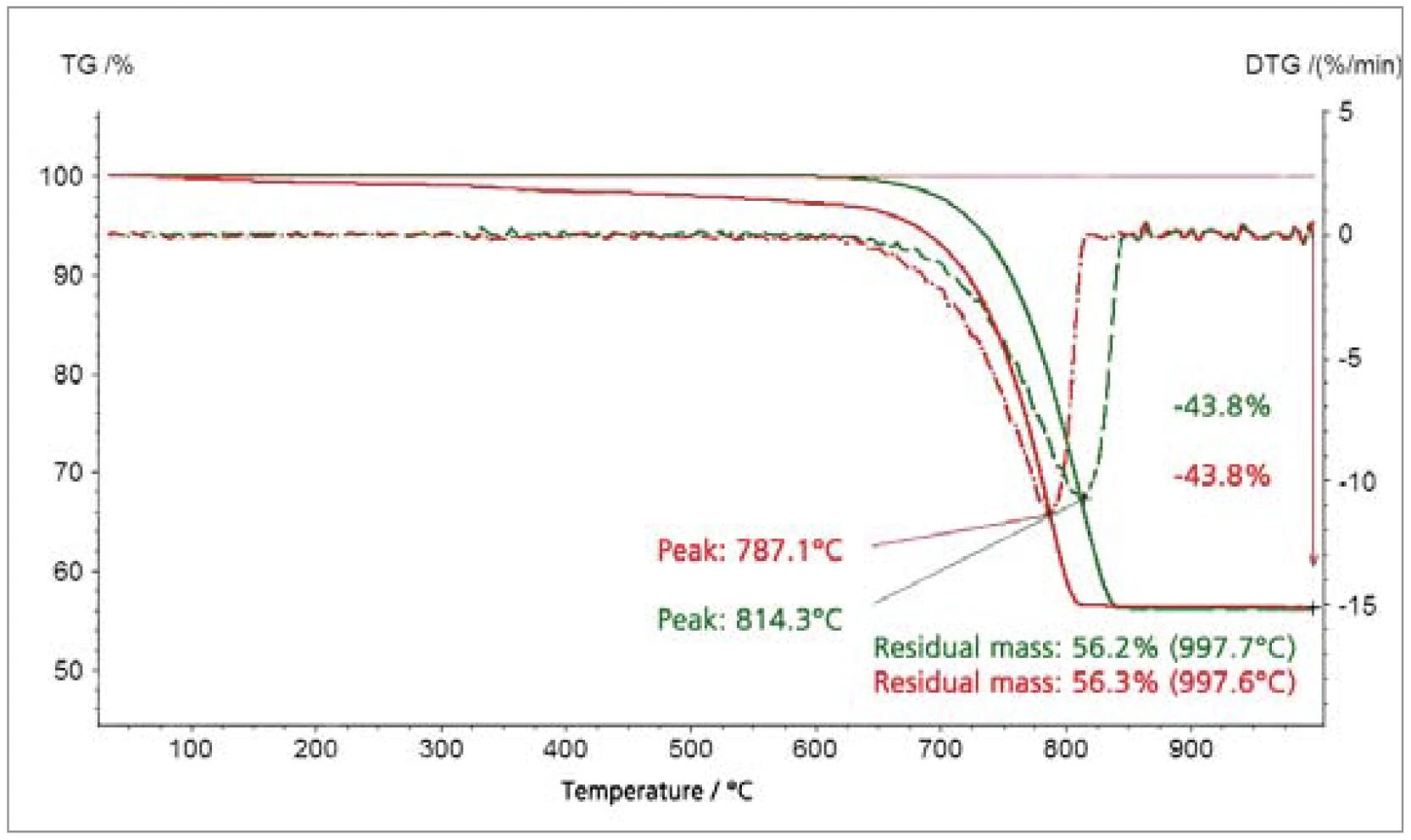
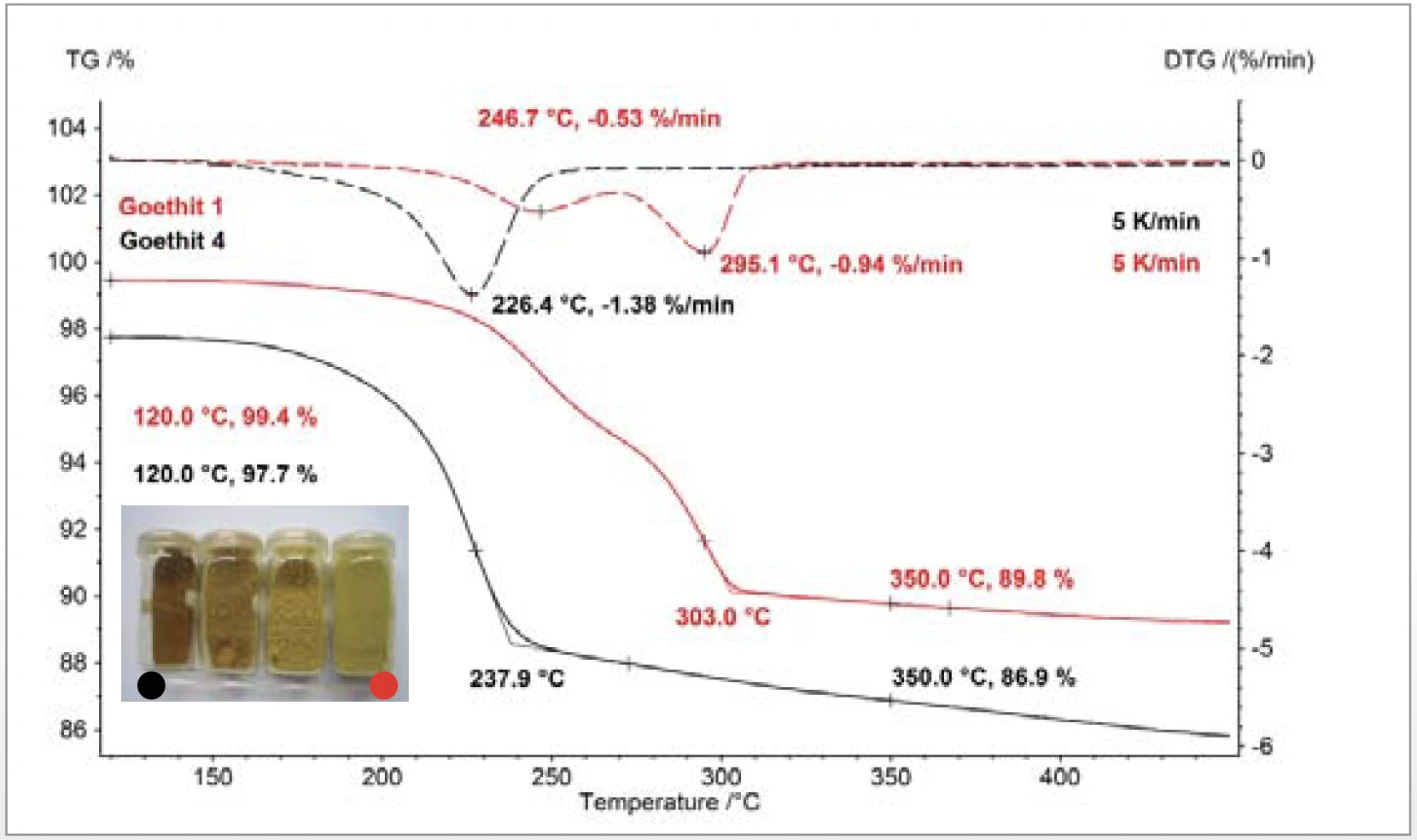
Sintering
The particle size-dependent effects observed during the SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering of pressed powder pellets can not be explained by increased surface area alone (figure 5). In contrast to melting behavior, particle size effects on SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering occur at dimensions as large as the micrometer range. Significant reductions in the SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering temperature occur with relatively small variation of the particle size.
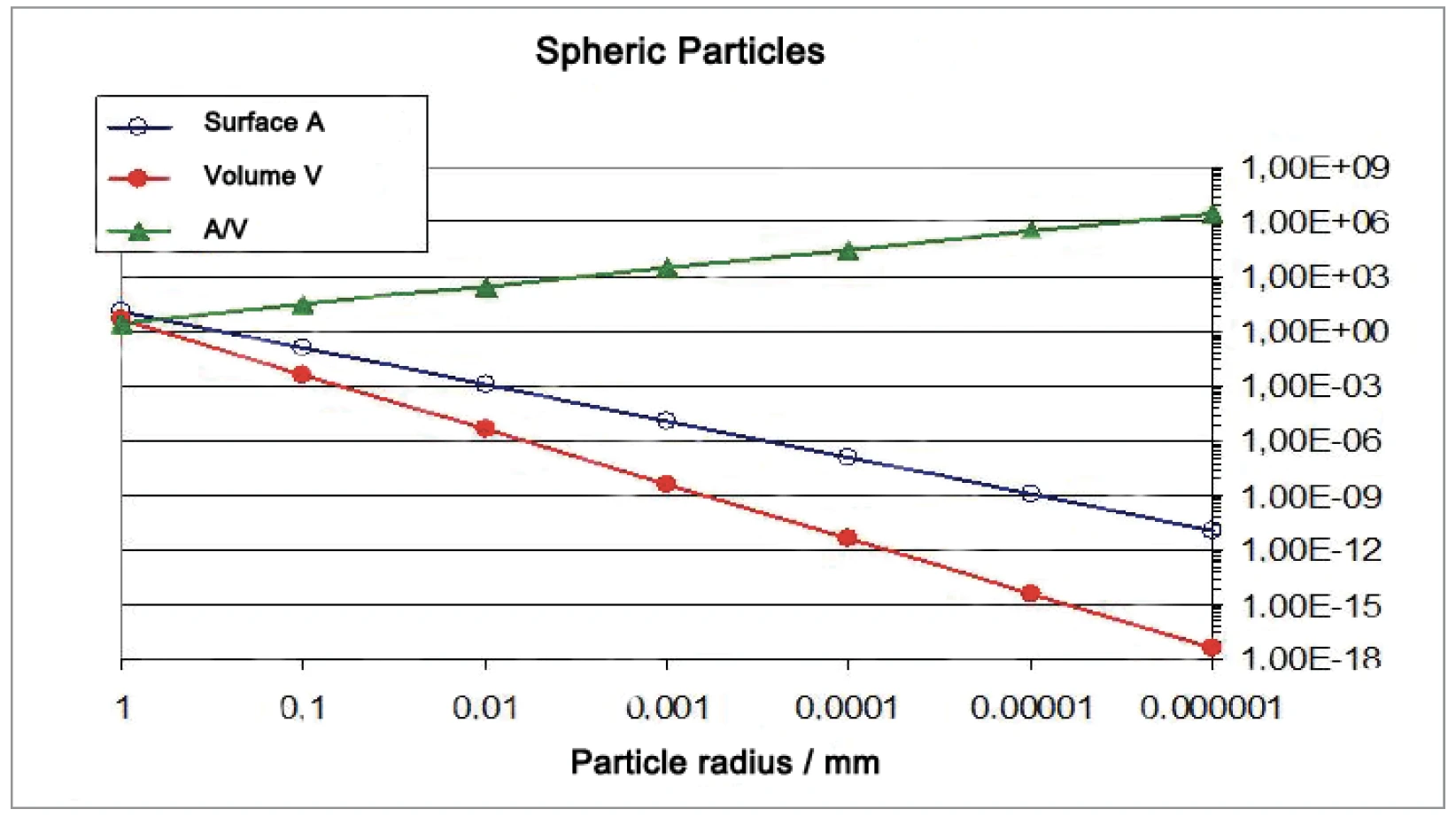
The amount of contact points between spherical particles increases much faster than the surface-to-volume-ratio (figures 6 and 7). For the increase in SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering activity, the contact points between the particles are important. Particles ranging from 10 μm to 130 nm in diameter were generated by grinding the materials with the NETZSCH ZETA® RS4 beat mill system.
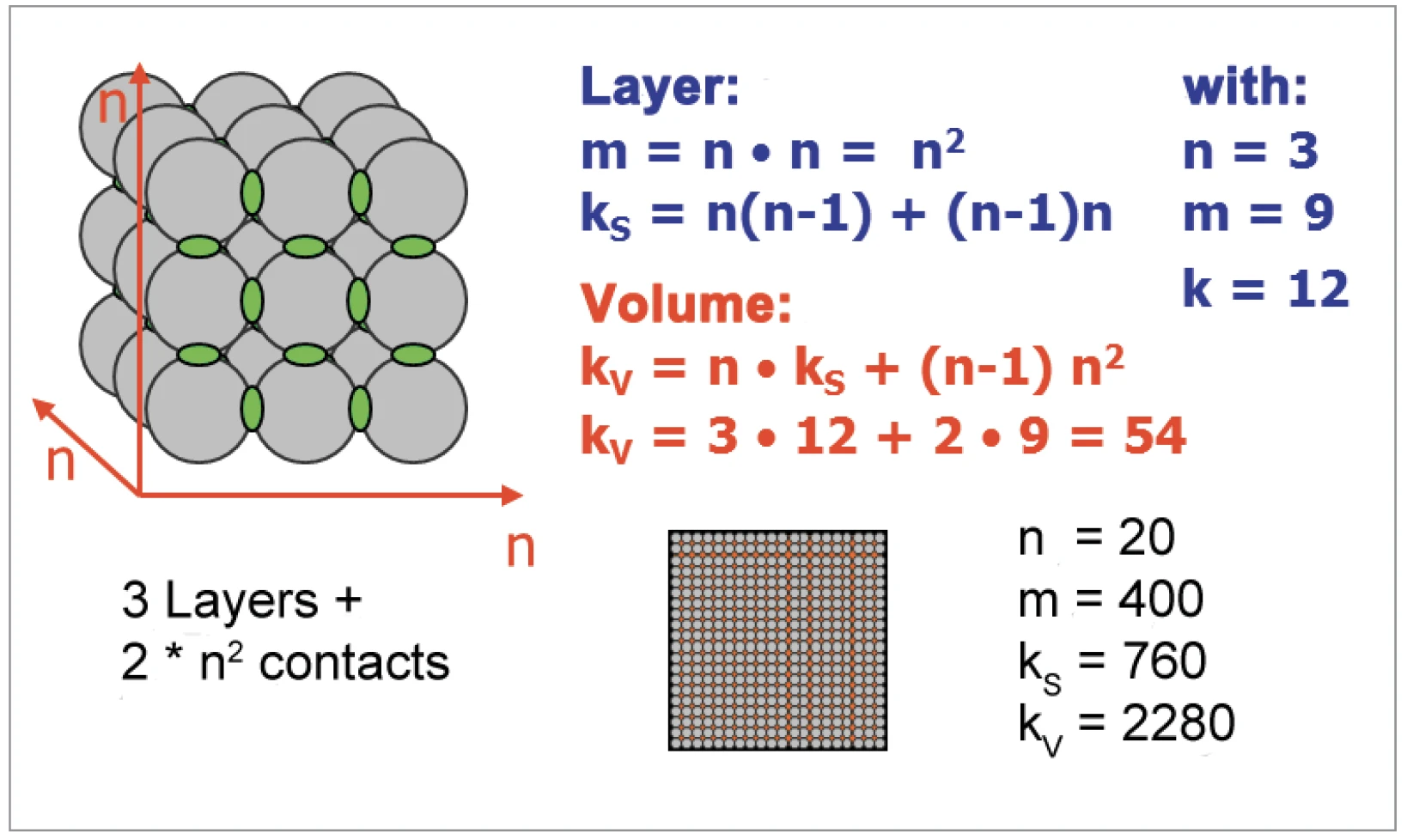
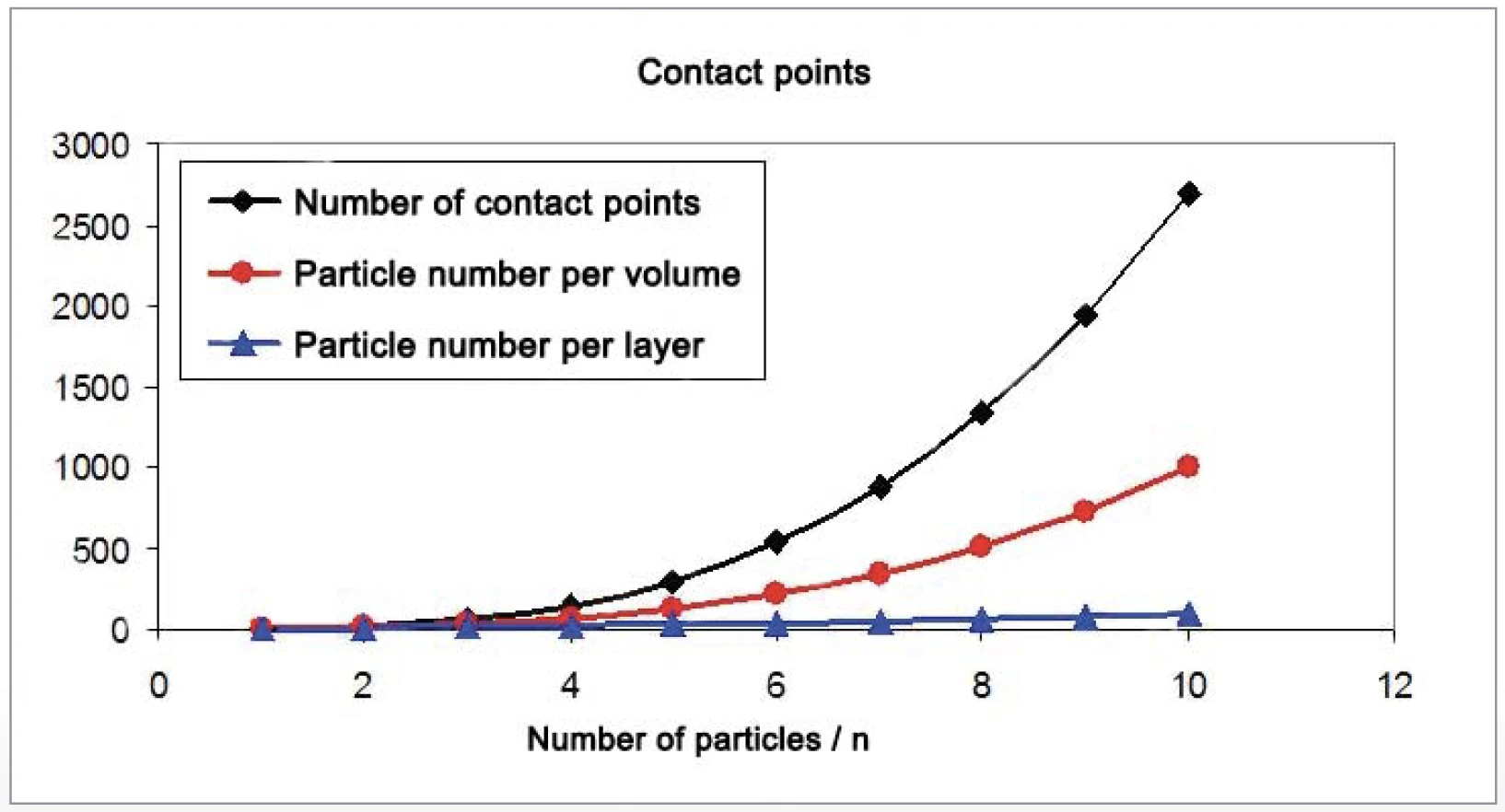
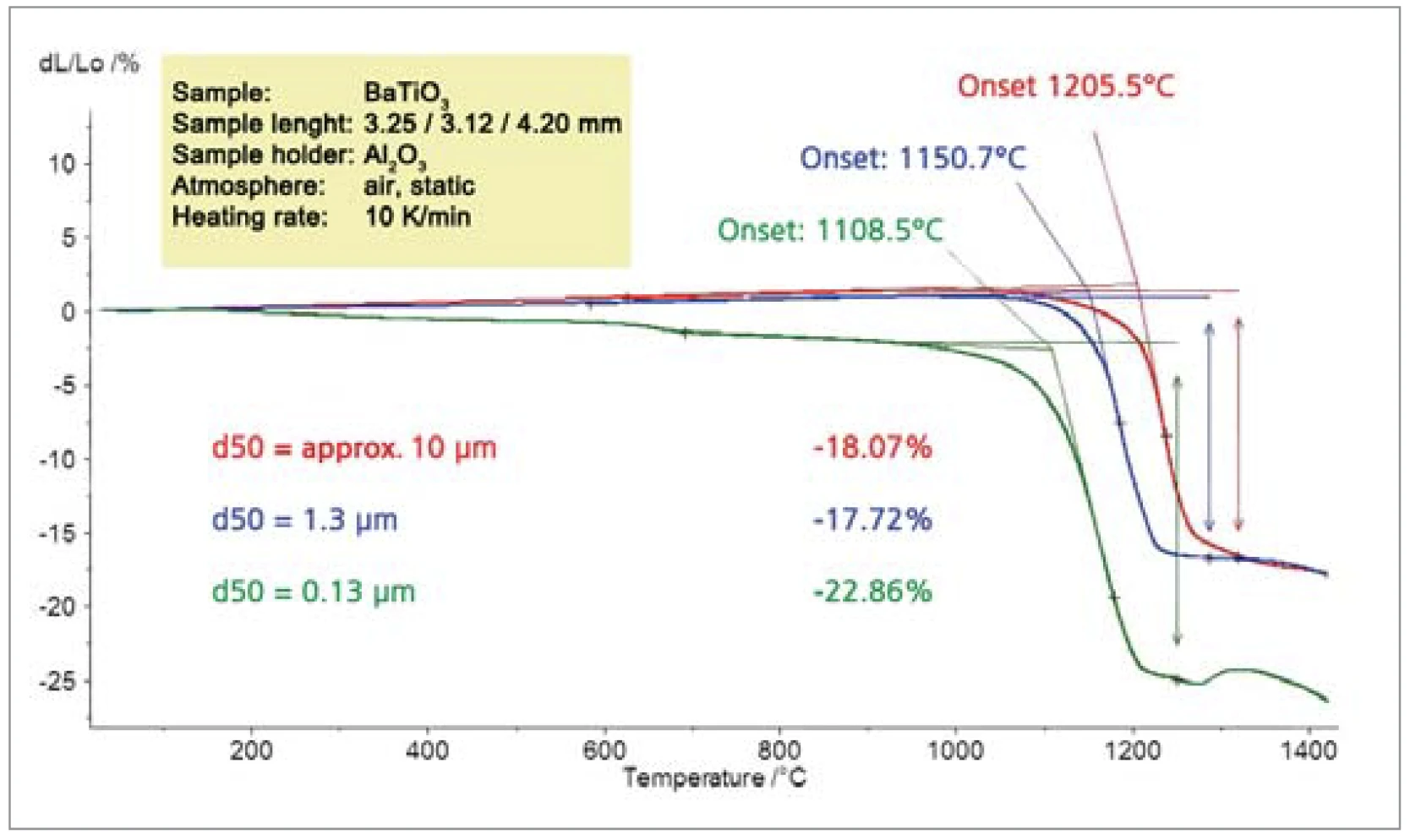
Figure 8 shows the SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering activity dependence on particle size for BaTiO3. The 1108°C SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering temperature for the smallest particles (extra-polated onset temperature) is almost 100 K lower than the SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering temperature of the larger particles (1205°C).
Summary
With the help of thermoanalytical measurements, it could be demonstrated that the particle size has a significant effect on the kinetics and, hence, temperature dependence of processes such as dehydration, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition, combustion and SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering. Sample preperation, particularly the particle size, is thus an important parameter to consider when interpreting measurement results.
Thermal analysis methods offer a relatively easy and quick means of measuring the effects of particle size on sample properties.