
20.02.2023 by Prof. Dr. Ing. Sascha Englich
DSC Analysis on Thermosets
DSC Analysis on Thermosets – Application of the Appropriate Measurement Methodology for Different Resin Types
Prof. Dr. Ing. Sascha Englich is a Professor for Plastics Engineering at the Steinbeis University of Berlin. As part of the new blog series for optimization of epoxy resin injection molding by means of differential scanning calorimetry and rheology, he already presented the reports on “Thermosetting Injection Molding in E-Mobility” as well as “Epoxy Resins – Reactive Polymers as a Basis for Injection-Moldable Compounds”.
In today’s article, you will learn more about Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing optimization by means of differential scanning calorimetry, DSC for short.
The basic chemical-physical functional principle of thermoset materials, i.e., the crosslinking of relatively short-chain molecular compounds to form a 3-dimensional molecular network, and the determination of the same by means of DSC analysis were already described in the blog article “Epoxy Resins – Reactive Polymers as a Basis for Injection-Moldable Compounds”. In principle, this also applies to all other industrially relevant thermoset materials, such as:
- Phenolic resins (PF)
- Unsaturated polyester resins (UP)
- Vinyl ester resins (VE)
- Melamine resins (MF)
- Urea resins (UF)
- Epoxy resins (EP)
In detail, however, there are differences between the individual types of thermosets, which have a significant influence on both processing and analysis. One of the reasons for this is the respective type of crosslinking reaction, analogous to the synthesis reactions of thermoplastics. A distinction is made between polyaddition, polymerization and polycondensation.
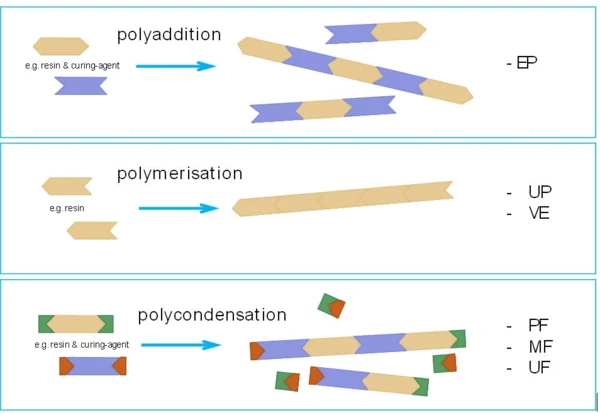
Not All Thermosets Are the Same
In Figure 1, the different chemical reaction principles are shown with a schematic using one-dimensional “molecular chains”. The most important and relevant difference is that polycondensation reactions always occur with the separation of low-molecular, volatile by-products under typical processing temperatures for thermosetting molding compounds. These separation products can, for example, be water or ammonia und must be taken strictly into account for both processing and analysis. Thermosets that crosslink by a polycondensation reaction include phenolic resins (PF) as well as amino resins (UF, MF, MP).
Another type of thermosetting molding compound also has volatile components; however, these do not result from the crosslinking reaction. These are so-called BMC and SMC materials (Bulk Molding Compound, Sheet Molding Compound). These are mostly compounds based on unsaturated polyester (UP) or vinyl ester (VE), to which a dough-like consistency is imparted by adding styrene compounds. These styrene compounds are partly polymerized, but are also partly lost as a volatile component.
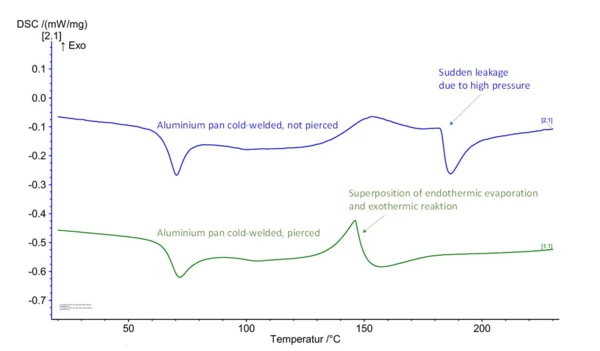
As indicated, volatile components must be taken into consideration during both processing and analysis. For DSC analysis, low-molecular, volatile components mean on the one hand phase transformation from liquid to gaseous – evaporation – during the measurement. This is measured as an endothermal effect in the heat-flow signal and would overlay the simultaneously occurring crosslinking reaction. Clear characterization of the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal reaction peak would thus not be possible. (Vgl. Fehler! Verweisquelle konnte nicht gefunden werden.). Apart from the measurement quality itself, volatile components which enter the measuring cell of the DSC instrument lead to severe contaminations. Figure 2 shows three different DSC crucible types used for the characterization of thermosetting molding compounds:

Which Crucible Is the Right One?
Cold-weldable aluminum crucibles/lids are typically employed for epoxy molding compounds (addition reaction without by-products), whereby the lid is usually additionally pierced. This prevents the thin lid from bulging due to the expansion of air in the crucible, which would result in an endothermal effect because of the increase in volume.
In the case of molding compounds that contain volatile components or release them during the crosslinking reaction ‒ these include phenolic resins and amino resins (condensation reaction with by-products) as well as BMC and SMC materials on a polyester and vinyl ester basis ‒ the use of cold-welded aluminum crucibles is not expedient. In an unpierced aluminum crucible, the condensation products cannot evolve at first, resulting in a steady pressure increase on the inside which also prevents evaporation. With increasing pressure, sudden leakage of the cold-welded crucible-lid connection occurs. In a pierced aluminum crucible, the condensation products can freely evaporate and escape. The endothermal evaporation enthalpy thus overlaps the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal crosslinking reaction. Meaningful evaluation of a reaction peak is not possible in either case (fig. 3).
This is the reason that pressure-tight steel crucibles are employed for these kinds of molding compounds. The tightly pressed crucibles with elastomer sealing are generally used. The pressure tightness of 20 bar is sufficient for the typical thermosetting molding compounds, since the resin content in the molding compound that produces the volatile components is usually low. Only the low upper temperature limit of 250°C (thermal application limit of the elastomer seal) can lead to insufficient measurement of the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal crosslinking peak at heating rates higher than 10 to 15 K/min. If, for example, higher heating rates are required for the determination/modeling of the reaction kinetics, the screwed steel crucibles can also be employed.
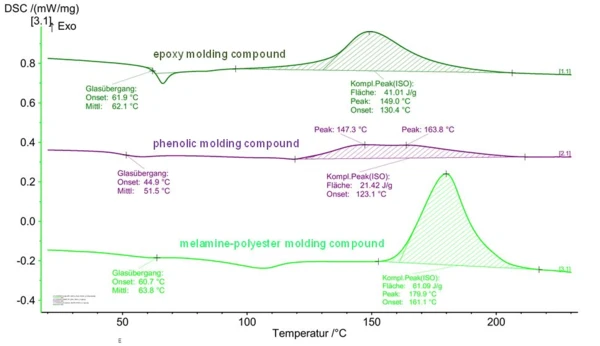
Figure 4 shows the example of DSC curves for different types of thermosetting molding compounds. The different “peak forms” for the crosslinking enthalpy can clearly be seen. Based on the peak evaluation, the principal behavior of processing/hardening can thus be derived. Onset and peak temperatures yield information on the reaction dynamics and, if required, the influence of catalysts or inhibitors on the onset of Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing (temperature) and the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing rate.
Using the peaks of the phenolic resin molding compound in Figure 4, it can also be seen that the measurable thermal effects (reaction enthalpy) are in part very low. The reason for this is the filling levels of thermosetting molding compounds, which are in part very high. In the example of this phenolic resin, it was a material with a resin content of “only” 20%. This fact should be considered during sample preparation by using higher sample quantities.
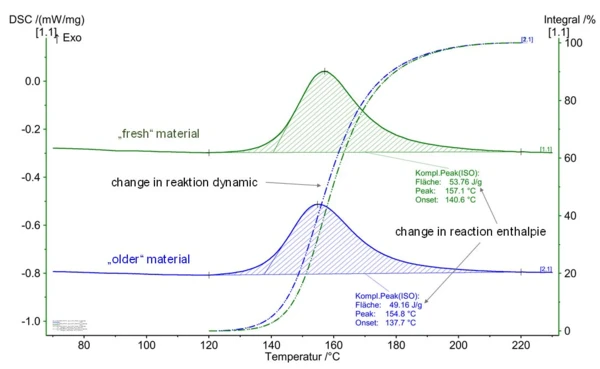
The reaction enthalpy can also be used to draw conclusions about the storage state of thermosetting molding compounds. The “storage state” can also be determined in the same way as the crosslinking state of building parts, as described in the blog article “Epoxy Resins – Reactive Polymers as a Basis for Injection-Moldable Compounds”. Figure 5 presents the comparative measurement on a “fresh” versus a “stored” epoxy molding compound. Changes with regard to both reaction dynamics and reaction enthalpy can clearly be seen.
Optimum Sample Preparation and Measurement Methodology
Taking all peculiarities typical of thermosetting molding compounds into account, the following criteria for both sample preparation and measurement methodology have proven to be appropriate (figure 6):
- Preparation of the granules to a fine powder, without thermal input if possible (e.g., mortaring)
- Utilization, if at all possible, of the entire crucible volume for the sample: a lot of reactive mass increases the signal strength
- Compacting of the sample in the crucible by the probe: good contact with the crucible bottom; little air as a thermal insulator in the sample
- Use of pierced aluminum crucibles for epoxy resins
- Use of tightly sealed steel crucibles for such substances as phenolic resins, amino resins, unsaturated polyester resins and vinyl ester resins
- Heating rates of 20 K/min for aluminum crucibles and 10 K/min for steel crucibles
- Implementation of a second heating (entirely hardened sample in the crucible) for baseline correction facilitates peak evaluation

In the next article, Prof. Dr. Ing. Sascha Englich will be reporting on kinetic simulation of the process. Stay tuned!

