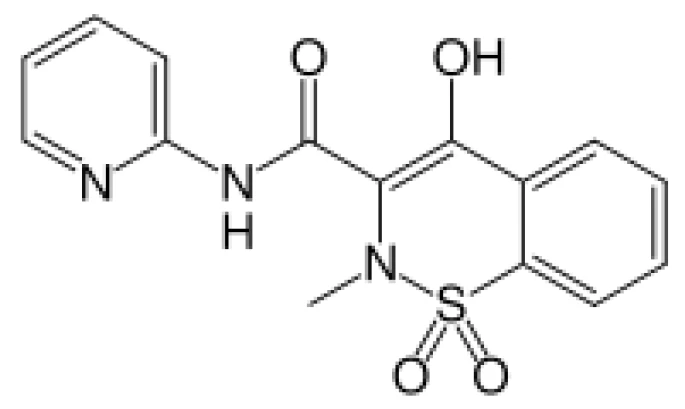
Introduction
The melting temperatures of various solids such as certain polymers, fatty acids, Ionic liquids, and pharmaceutical compounds have been found to be lowered substantially in the presence of supercritical carbon dioxide (scCO2) due to the solubility of the CO2 in the melt. This effect can be beneficial for processing or crystallizing the materials from their melts, especially if they are thermally sensitive. The susceptibility of a material to Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point depression in the presence of scCO2 can be explored by using high pressure DSC (HP-DSC) to measure the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of the material under elevated pressures of CO2, even without reaching supercritical conditions. In this study, the effect of high pressures of carbon dioxide on the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of the pharmaceutical compound piroxicam, a non-steroidal anti-inflammatory drug (NSAID), was investigated. Four anhydrous crystalline forms (polymorphs) of this compound have been reported [1]. Commercially available form I, with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of ca. 201°C, is the most stable crystalline form. The Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of form I has been shown to be depressed substantially in the presence of scCO2 [1]. Since piroxicam decomposes upon melting, lowering the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of the compound could be beneficial for growing other crystalline forms (e.g., form III) from the melt that are difficult to access by CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization from solutions in organic solvents.
Experimental Details
Piroxicam (TCI America) was used as received. DSC measurements were performed with the NETZSCH DSC 204 HP Phoenix® on 4-6 mg samples in open 25 μL aluminum crucibles. Samples were heated at 10 K/min under a flow of N2 or CO2 with pressures ranging from 1 to 40 bar at a flow rate of 100 mL/min or under a static CO2 atmosphere for achieving pressures of >55 bar. An indium standard was used to verify the temperature calibration of the instrument, which did not vary under the different atmospheres and pressures.
Results
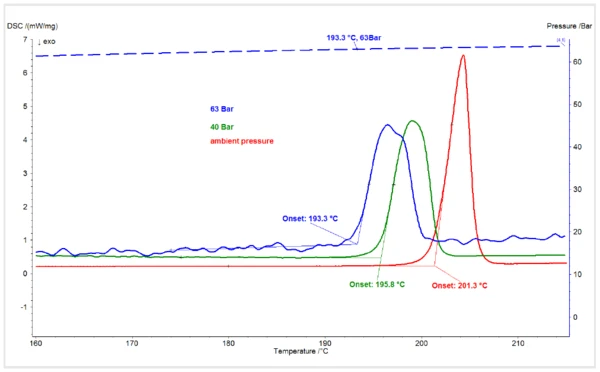
Figure 1 shows the melting transitions in the DSC curves of piroxicam form I under ambient CO2 pressure and CO2 pressures of 40 bar and 63 bar. Whereas measurements at ambient pressure and 40 bar were performed under a dynamic flow of CO2, the measurement at 63 bar was performed under a static atmosphere of CO2 in a closed system. The maximum pressure of 55 bar from the CO2 tank was admitted to the HP-DSC at ambient temperature, and the system was closed so that the pressure increased with heating, reaching 63 bar at the onset of sample melting. The extrapolated onset temperature of the piroxicam melting peak of 201.3°C at ambient pressure is consistent with the literature value1. The melting onset was depressed by approximately 5.5 K to 196°C under 40 bar CO2. It decreased by an additional 2.5 K under a CO2 pressure of 63 bar.
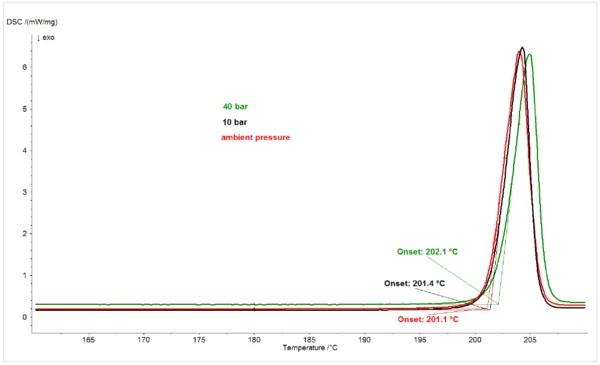
To verify that the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point depression effect on piroxicam by increasing CO2 pressure was specific to CO2, the effect of increasing N2 pressure on the melting behavior of the compound was examined. Figure 2 shows the melting peaks of piroxicam under N2 at ambient pressure, 10 bar, and 40 bar. In contrast to the melting point depression effect of increasing CO2 pressure, increasing N2 pressure caused a slight increase in the melting point of piroxicam, consistent with the behavior of most materials, which undergo expansion when changing from solids to liquids.
Summary
HP-DSC measurements showed that piroxicam undergoes a melting point depression of approximately 8 K in the presence of a CO2 atmosphere of 63 bar compared to the situation at ambient pressure. This study demonstrated the utility of HP-DSC measurements for screening solids for potential melting point depression in scCO2, even when the pressures are below what is necessary to reach the supercritical phase.