Introduction
In Application Note 032, we presented the ways in which the atmosphere and sample shape aff ect the TGA test results using detailed examples from the field of thermoplastic elastomers. In addition to the purge gas type (e.g., inert or oxidative), other factors infl uencing the test results are the purge gas rate, the influence of the crucible, the surface-to-mass ratio and if the measurements were carried out using open or closed crucibles.
Measurement Results
Influence of the Purge Gas Rate
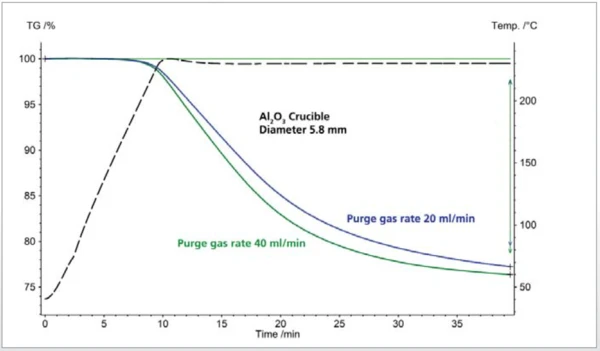
Figure 1 shows the TGA curves for a polymer additive formulation at two different purge gas rates. The solid sample was heated under nitrogen to 230°C and the temperature was then held constant. During heating, the sample melted at 75°C and then remained in the liquid state.
For both measurements, a sample weight of 10.50 mg and a heating rate of 20 K/min were used. The mass loss observed here is higly correlated with the purge gas rate employed. For the purge gas rate of 40 ml/min (green TGA curve), a mass loss of 23.6% can be observed; for the lower purge gas rate of 20 ml/min (blue TGA curve), the mass loss amounted to only 22.8% after the same amount of time. The mass-loss step in this example cannot be attributed to Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the sample, but rather to the evaporation of volatiles. The evaporation process can thus be accelerated by means of high purge gas rates.
Influence of the Crucible, Surface-to-Mass Ratio
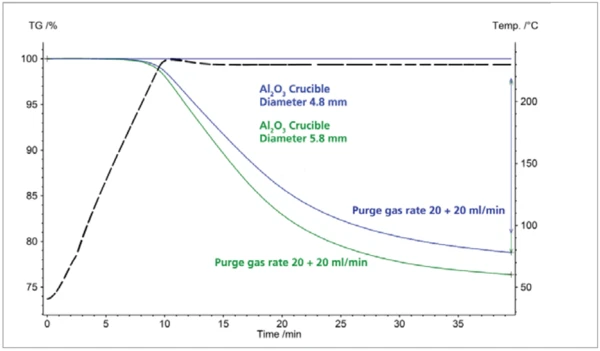
Similarly, the choice of crucible also affects the TGA test result. Figure 2 shows the test results for the same sample shown in figure 1. All measurement parameters – temperature program, sample weight, atmosphere – were set identically. The only diff erence was the crucible geometry. For the test yielding the blue TGA cruve, the crucible had a smaller diameter than for the one yielding the green curve. Also a clear difference in the mass-loss step can be seen in the TGA test here.
With the larger crucible (green curve), a mass loss of 23.6% was observed; for the smaller crucible, the mass loss amounted to only 21.2% under otherwise identical measurement conditions. In thermal analysis, the ratio of surface to sample mass always plays a decisive role in the reproducibility of thermogravimetric test results.
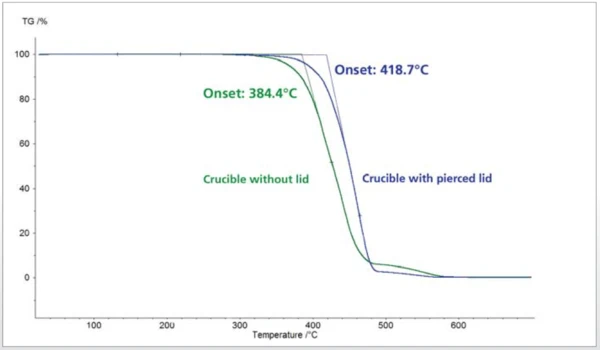
Measurement with and without Crucible Lid
Besides the crucible geometry, another important consideration when comparing results is whether a lid was employed for the measurements. Generally, TGA measurements are carried out without a lid, but sometimes a lid is used to prevent the sample material in a liquid state from spilling out of the crucible. In cases such as these, a closed lid is generally used. Figure 3 depicts the diff erence in Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition behavior of an HDPE sample as measured in an Al2O3 crucible without a lid and versus in a closed crucible with pierced lid. The sample weight for both measurements was 10 mg and the heating rate amounted to 10 K/min. The measurements were carried out under a synthetic air atmosphere. Under these measurement conditions, it can be assumed that the polyolefin is undergoing thermo-oxidative Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition. The oxygen contained in the purge gas (synthetic air) is simultaneously the reaction partner for the sample. The concentration of oxygen at the sample thus has a direct influence on the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition itself and/or the beginning of Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition. This can be evaluated using the extrapolated onset temperatures in figure 3. In the measurement without a lid, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition starts already at 384°C; in the measurement with a pierced lid, on the other hand, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition does not occur until 419°C. In the measurement with pierced lid, the sample does not come into contact with oxygen until a later point in time, so OxidationOxidation can describe different processes in the context of thermal analysis.oxidation is not observed. The residual mass, however, is unaffected by this and is identical in the two measurements.