Introduction
The Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of a material can only be determined with differential scanning calorimetry in dynamic measurements. A temperature-modulated measurement, in which the sample is no longer subjected to only a linear heating rate, but also to sinusoidal temperature variations, offers the ability to measure the heat capacity of the sample during IsothermalTests at controlled and constant temperature are called isothermal.isothermal segments. In what is known as a “quasi-IsothermalTests at controlled and constant temperature are called isothermal.isothermal” modulated DSC measurement, a sinusoidally varying temperature program is applied to a test specimen around an underlying IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature (definition from ASTM E2716-09).
In the following, the heat capacity of a sapphire sample was investigated with the DSC 214 Polyma by means of quasi-IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature modulated measurements at different temperatures. The tests were carried out on the basis of method B of ASTM E2716-09.
Test Results
The measurements were carried out with Concavus® crucibles with pierced lids. A sapphire disk of 49.88 mg was employed as the calibration material. The sample had a mass of 50.41 mg.
The temperature program included cooling and heating at a controlled rate of 10 K/min to the desired temperature. During the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment of 10 minutes at this specific temperature, oscillations of periods of 100 s or of 1 K amplitude were used for modulation, as recommended in ASTM E2716-09. This procedure was repeated 11 times between 0°C and 100°C with an increase of 10°C for each step (i.e., IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperatures of 0°C, 10°C, etc., up to 100°C).
The heat capacity results of the sample for all 11 quasiisothermal segments between 0°C and 100°C are depicted in figure 1.
The determined results for the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of sapphire were compared to the literature data for sapphire (see table 1).
The specific heat values determined with the DSC 214 Polyma are in very good agreement with the literature values (maximum error: 1.82%).
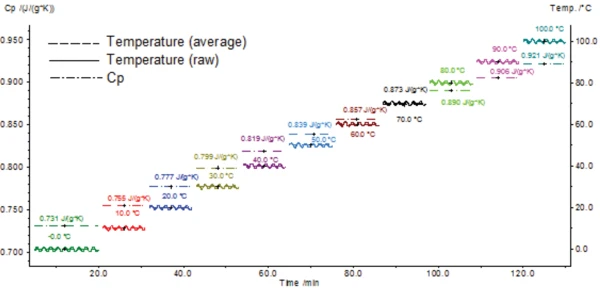
Table 1: Comparison of the results for the specific heat capactiy of sapphire compared to literature data
Temperature [°C] | Specific heat of sapphire [J·g-1·K-1] | Specific heat measured with DSC 214 Polyma [J·g-1·K-1] | Difference between literature and measured values [%] |
|---|---|---|---|
| 0 | 0.718 | 0.731 | 1.82 |
| 10 | 0.742 | 0.755 | 1.80 |
| 20 | 0.764 | 0.777 | 1.68 |
| 30 | 0.785 | 0.799 | 1.72 |
| 40 | 0.806 | 0.819 | 1.66 |
| 50 | 0.825 | 0.839 | 1.72 |
| 60 | 0.860 | 0.857 | -0.36 |
| 70 | 0.876 | 0.873 | -0.39 |
| 80 | 0.892 | 0.890 | -0.21 |
| 90 | 0.907 | 0,906 | -0.06 |
| 100 | 0.920 | 0.921 | 0.06 |