Introduction
Caffeine is the main active ingredient in coffee. However, there are some circumstances – such as increased blood pressure, sensitive stomach, pregnancy or interaction with pharmaceuticals – under which decaffeinated coffee is preferred. In 1903, the first procedure to decaffeinate coffee beans was developed. This procedure used water and benzene to extract caffeine from the raw beans (Roselius method). Later, other solvents such as dichloromethane or ethyl acetate were used to substitute the carcinogenic benzene. Nowadays, new processes such as pure water extraction (Swiss water process), the triglyceride process or extraction by supercritical carbon dioxide are common. [1]
However, decaffeinated coffee still contains some residual caffeine. Pure raw coffee beans contain between 0.8% and 4% caffeine, depending on the type of coffee bean [2]. The EU allows a residual amount of 0.1% caffeine in the unroasted beans when selling a decaf coffee [3]. The amount of caffeine which is transferred from the bean to the cup of brewed coffee depends on the amount of coffee grounds used, the kind of roasting, the grain size of the coffee grounds after milling, the extraction time and pressure, and the water temperature. This results in an amount of about 3 mg of caffeine per cup (150 ml) coffee for decaf coffee, whereas a normal cup of coffee contains between 50 and 100 mg caffeine. The caffeine content in instant coffees is also less than for normal coffee. Depending on the kind of bean used and the production process, instant coffee only contains about 50% of the caffeine found in a filter coffee. Mean values range between 30 and 90 mg per cup of 150 ml. Producers of instant decaf state that their products contain less than 5 mg caffeine per serving.
The main difference between instant coffee and brewed coffee is the preparation. Like regular coffee, instant coffee comes from beans that are ground and brewed, but then freeze-dried or spray-dried into a concentrated powder. This means that once the powder is mixed with water, it will regain normal coffee flavor and texture.
In this study, different instant coffees with and without caffeine were investigated by means of TGA-GC-MS (Thermogravimetry gas chromatography mass spectrometry, STA 449 F3 Jupiter® coupled to Agilent GC 8890 and Agilent MDS 5975) to determine the caffeine contained.
For sample preparation, the samples were slightly ground and compressed in the crucible; then transferred into the STA. The TGA measurements were baseline-corrected. The samples were heated in an inert atmosphere to 850°C to evolve volatile compounds such as caffeine. The evolved compounds were collected on the GC cryo trap at -50°C, then separated and identified after the TGA run.
Table 1: TGA measurement parameters
| Sample | 1 (freeze-dried) | 2 (spray-dried) | 3 (freeze-dried) | 3a decaf (freeze-dried) | Pure caffeine | |||
| Sample mass | 7.26 mg | 7.13 mg | 7.46 mg | 7.38 mg | 10.39 mg | |||
| Crucible | Open Al2O3 crucible (85 μl) | |||||||
| Sample carrier | TGA, Type S + slip-on plate | |||||||
| Furnace | SiC | |||||||
| Temperature program | RT - 850°C | |||||||
| Heating rate | 10 K/min | |||||||
| Gas atmosphere | Helium | |||||||
| Gas flow (total) | 70 ml/min | |||||||
Table 2: GC-MS measurement parameters
Cryo Trap Mode | ||||||||
| Column | Agilent HP-5ms | |||||||
| Column length | 30 m | |||||||
| Column diameter | 0.25 mm | |||||||
| Cryo trap temperature | -50°C, 81 min | |||||||
| Column temperature | 45°C, 83 min isotherm 45°C to 300°C, 10 K/min | |||||||
| Gas | Helium | |||||||
| Gas flow (split) | 20 ml/min (10:1) | |||||||
| Valve | each 1 min | |||||||
Results and Discussion
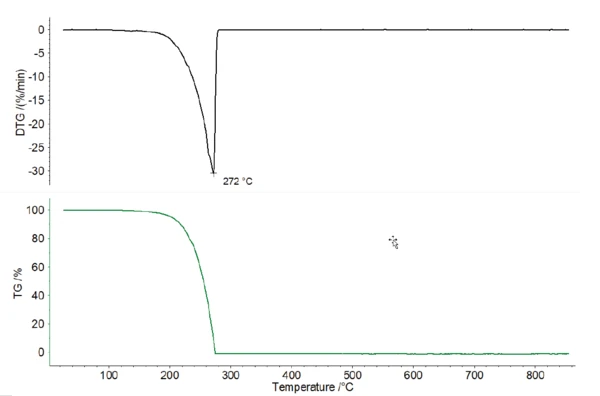
All of the coffee samples showed several significant mass-loss steps between room temperature and 850°C; see figure 1. It was not possible to clearly separate the mass-loss steps with the applied heating rate of 10 K/min. All four samples showed relatively similar behavior. Additionally, it was not possible to clearly identify the caffeine release through TGA alone. Pure caffeine shows a DTG (mass-loss rate) peak at 272°C (see figure 2), which was overlapped by other effects in the coffee samples.
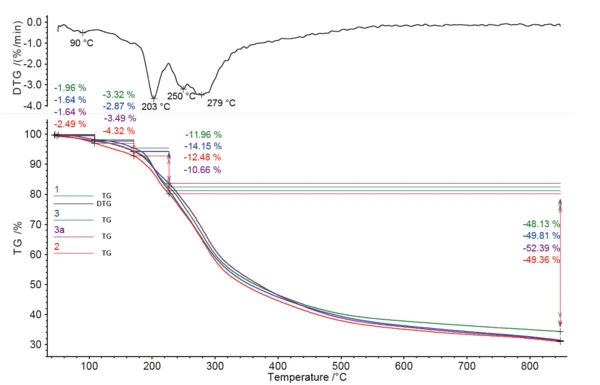
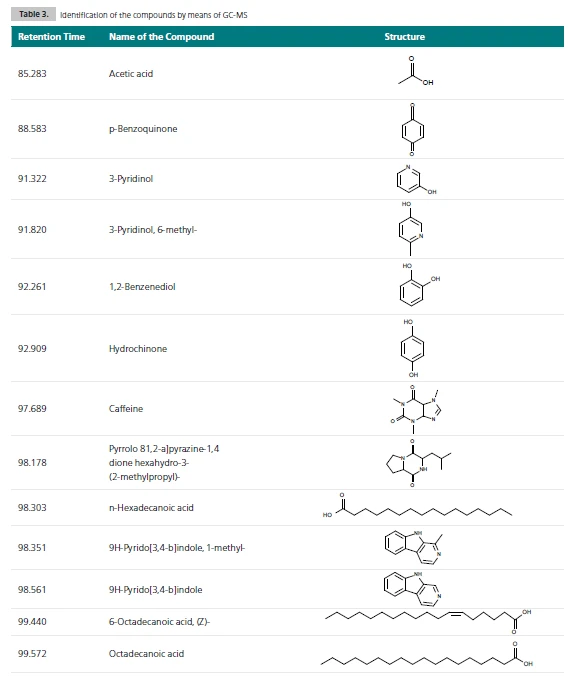
The GC-MS technique is necessary in order to separate the compounds released and to identify the caffeine in this complex mixture. The resulting total ion current shows a number of gaseous compounds detected for each sample; see table 3.
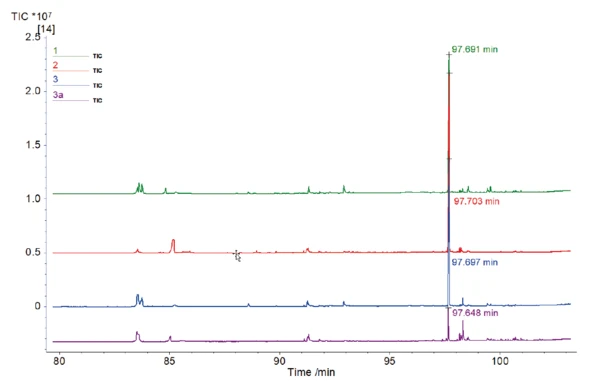
With the help of the NIST library, the main peak of each chromatogram can be related to caffeine; see figure 3. It was shown that high-boiling substances such as caffeine can be transferred to the MS without condensation.
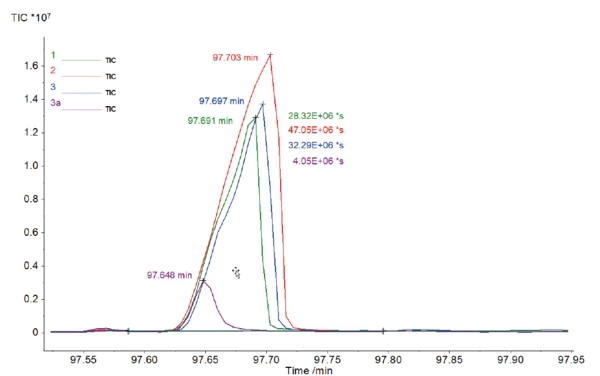
A closer look at this peak (figure 4) with a retention time of about 98 min shows the different sizes and consequently the different areas below these peaks. The area below the peak can be compared relatively and is related to the amount of caffeine contained. The mean area for all caffeinated coffee samples yields a value of 35.88·106*s. The result for the decaf sample was 4.05·106*s. With the relative comparison of these values, it can be estimated that in this case, the decaf sample contains less caffeine than the regular samples by about a factor of 9.
The amount of caffeine per cup heavily depends on the production procedure for instant coffee and the amount of coffee powder used per cup. Serving suggestions range between 2 and 4 g per cup.
Summary
The technique of TGA-GC-MS offers a great variety of different options for analyzing and comparing different food products. Continuous high heating of the transfer systems allows for the condensation-free transfer of high-boiling point substances. In addition to identifying the different organic compounds released, it was also possible to carry out a relative comparison of the caffeine contained. Although one sample was decaffeinated, residual caffeine was still detected. This demonstrates that GC-MS is a very sensitive system for detecting traces of gases released in the μg range.