Introduction
Lactose is a disaccharide sugar composed of galactose and glucose that is found in the milk of mammalians. Lactose makes up around 2% to 8% of milk (by weight), although the amount varies by species and among individuals. The name comes from lac (gen. lactis), the Latin word for milk, plus the -ose ending used to name sugars [3].
Lactose is frequently used in food technology or as an excipient in pharmaceutical products. Knowledge of the thermal properties of lactose is essential because its Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition is in direct relation with the physical properties – such as stickiness and flowability – of powders containing milk sugar, and this in turn influences the processing. [4]
In the following, the influence of the heating rate on the thermal properties of α-lactose monohydrate FlowLac® 90 supplied by MEGGLE was investigated by means of DSC. As a spray-dried product, it typically exhibits an amorphous content of 10% to 15%. [5]
Test Conditions
The measurements were carried out with the NETZSCH DSC 214 Polyma in a dynamic nitrogen atmosphere. The samples of masses between 4.21 mg and 4.74 mg were weighed in Concavus® aluminum crucibles which were sealed with a pierced lid and heated to 280°C at different heating rates (20, 50, 100 and 200 K/min).
Test Results
Figures 2 and 3 depict the DSC measurement curves for the different heating rates.
A change in heat capacity with a midpoint between 62°C (measurement at 20 K/min) and 85°C (measurement at 200 K/min) indicates the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of the sample. The endothermal peak detected between 148°C and 185°C (peak temperature) comes from the release of the water. This is in accordance with the results published in [2] that lactose monohydrate released its hydrate water when heated above 150°C.
The second peak, located between 222°C and 248°C, is due to the melting of the α-lactose anhydrate crystals. Although the course of the curves is very similar, the influence of the heating rate can be seen on all effects (Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition, dehydration and melting). First, they are shifted to higher temperatures with increasing heating rates. Second, increasing the heating rate leads to an amplification of the DSC effects. This is due to the influence of the heating rate on the kinetics of the processes.
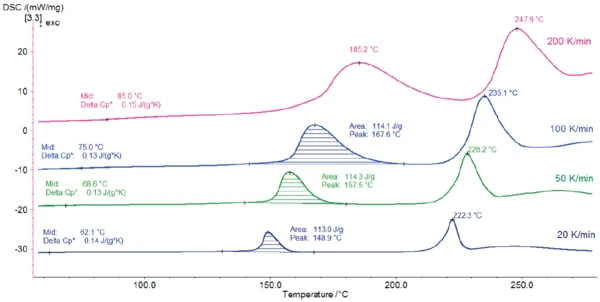
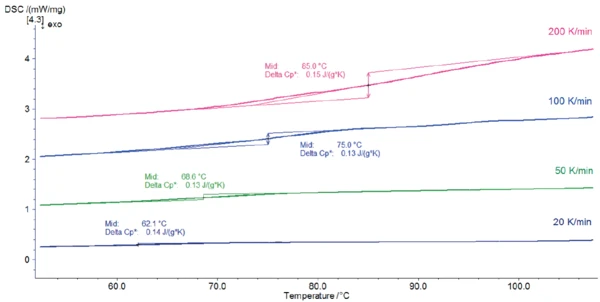
Increasing the heating rate is useful for improved detection of small effects. In this example, for instance, the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of lactose is easier to detect in measurements carried out at higher heating rates. On the other hand, decreasing the heating rate helps separate overlapping effects. In the measurement at 200 K/min, the peak of the water release is partially overlapped with the melting peak at 248°C, which makes evaluation of the peak enthalpy difficult. In contrast, the energy of dehydration can be precisely determined for lower heating rates.
Conclusion
The thermal effects of α-lactose monohydrate can be easily determined by means of differential scanning calorimetry (DSC). TheGlass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery. glass transition temperature as well as the dehydration and melting peaks are dependent on the heating rate.
For an improved evaluation, increasing the heating rate can be a helpful tool when small effects in the DSC curve need to be amplified, and decreasing the heating rate can help when overlapped effects need to be separated.