Introduction
The methods of thermogravimetry (TGA) are particularly well suited for the investigation of combustion processes. They allow for rapid conclusions regarding the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability of the mostly solid fuel, as well as the reaction temperature and combustion kinetics. Furthermore, both the mass loss during a combustion reaction and the non-combustible mineral Ash ContentThe ash is a measure of the mineral oxide content on a weight basis. Thermogravimetric analysis (TGA) in an oxidative atmosphere is a well-proven method to determine the inorganic residue, commonly referred to ash, in organic materials such as polymers, rubbers, etc. Therefore, the TGA measurement will identify if a material is filled and calculates the total filler content.ash content can be quantified. Contrary to other reactions such as Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition or the release of humidity or solvents, combustion is a solid-gas reaction. Therefore, not only must all of the customary parameters such as sample mass, heating rate, and purge gas flow be kept constant, but the measurement results are also influenced by the sample surface, the concentration of oxygen and the crucible geometry, all of which can limit access to the solid sample by the reaction gas.
To pursue this issue, a series of measurements was carried out with a NETZSCH STA using different crucible geometries under otherwise identical conditions. The different crucibles are shown in figures 1 and 3; among them is also a pierced DTA crucible which is shown on an enlarged scaling in figure 2 [1].


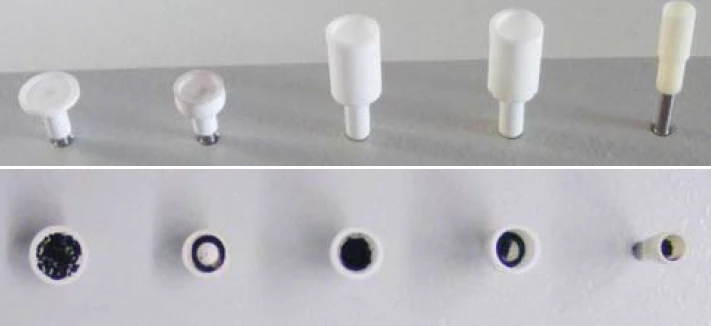
The investigated Carbon BlackTemperature and atmosphere (purge gas) affect the mass change results. By changing the atmosphere from, e.g., nitrogen to air during the TGA measurement, separation and quantification of additives, e.g., carbon black, and the bulk polymer can become possible.carbon black samples are different standards samples such as NIST 2975, Printex 90, activated carbon and carbon balls. These have a diameter of approximately 1 mm to 2 mm and an inorganic structure. The mean particle size of the powder samples is indicated between 20 nm and 50 nm.
Results
For the investigation of Carbon BlackTemperature and atmosphere (purge gas) affect the mass change results. By changing the atmosphere from, e.g., nitrogen to air during the TGA measurement, separation and quantification of additives, e.g., carbon black, and the bulk polymer can become possible.carbon black NIST 2975, the crucible types presented in figure 1 were employed. The relationship between the crucible diameter and the filling level of the samples (for the same sample mass) can be seen in figure 3 and table 1.
Table 1: Dimensions of the crucibles shown in in figure 1
Dimensions (mm) | Slip-on plate | Short DTA crucible | DTA crucible | DTA crucible, pierced | Mini DTA* |
|---|---|---|---|---|---|
| Ø outer | 10 | 8 | 8 | 8 | 5 |
| Ø inner | 10 | 6 | 6 | 6 | 4 |
*for comparison only; this crucible is not part of the NETZSCH crucible product assortment
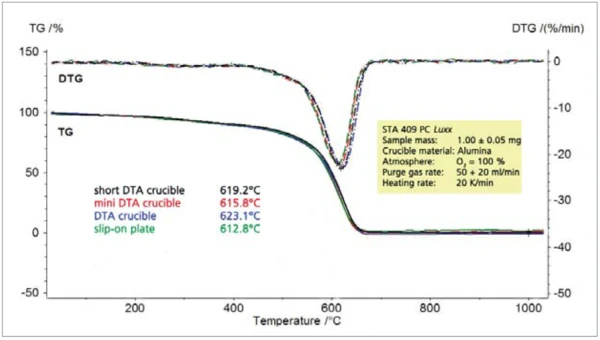
When using oxygen as a purge gas, small differences between the various crucible geometries can already be found with regard to the combustion temperature and with regard to the rate of combustion (DTG) (figure 4).
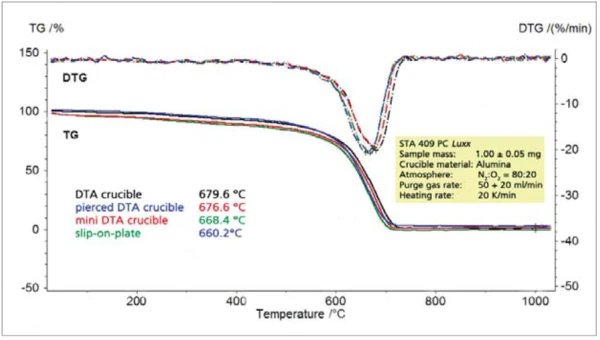
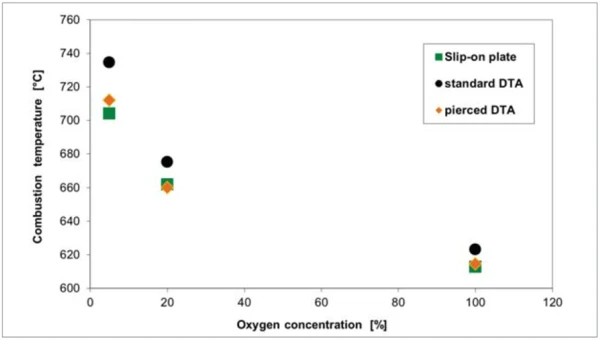
If, however, the concentration of oxygen in the purge gas is reduced to 20% (figure 5) or 5% (figure 6), the crucible geometry appears to play an increasingly important role. The pierced DTA crucible and the slip-on plate obviously allow for a better access of the reaction gas oxygen to the sample. However, the poorer the access of the reaction gas to the solid sample, the greater the tendency for the reaction to shift to higher temperatures and the lower the rate of reaction (DTG). At a nitrogen-tooxygen purge gas ratio of 95:5, the pierced DTA crucible is nearly as “fast” as the slip-on plate. With regard to the reaction behavior, the pierced DTA crucible (figure 2) and the short DTA crucible come closest to the slip-on plate, whereby sample handling for these two crucible types is significantly easier than for the slipon plate.
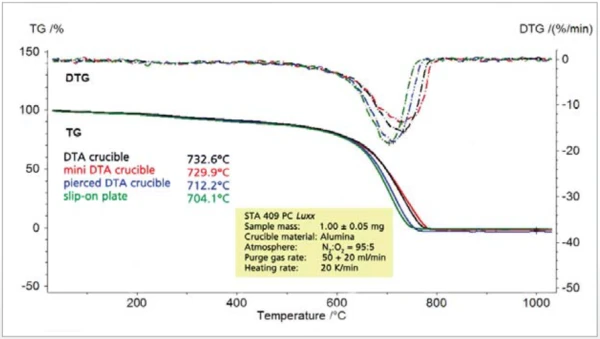
The dependence of the results on the oxygen content in the purge gas is illustrated in figure 7.
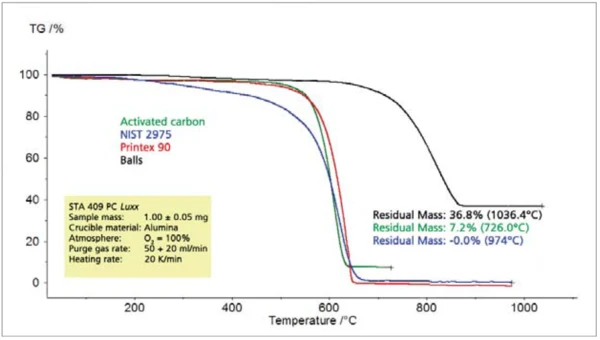
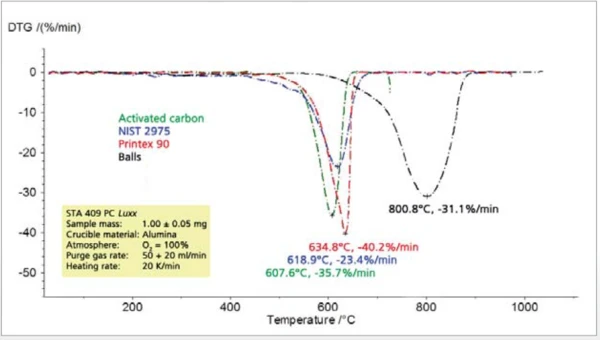
The comparison of different types of Carbon BlackTemperature and atmosphere (purge gas) affect the mass change results. By changing the atmosphere from, e.g., nitrogen to air during the TGA measurement, separation and quantification of additives, e.g., carbon black, and the bulk polymer can become possible.carbon black shows significant differences among all of the characteristic values to be determined such as Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability, combustion temperature, rate of combustion and residual mass (figures 8 and 9).
Conclusion
The measurements presented show that the crucible geometry can have significant influence on the interaction between the sample and the purge gas. The combustion reaction of Carbon BlackTemperature and atmosphere (purge gas) affect the mass change results. By changing the atmosphere from, e.g., nitrogen to air during the TGA measurement, separation and quantification of additives, e.g., carbon black, and the bulk polymer can become possible.carbon black was used here as an example. Under otherwise identical measurement conditons, as long as the same crucible type was employed within one test series, a comparative evaluation of the samples was possible. The effect of basic measurement conditions, including crucible type, on reaction rate must always be considered when performing kinetic studies. In this case, the slipon plate and pierced crucible proved suitable.