Introduction
Until now, IsothermalTests at controlled and constant temperature are called isothermal.isothermal CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization of polyolefines has not been easy to measure in heat-flux DSCs because of the fast reactions. If the IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature of CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization is not achieved fast enough, the polymer crystallizes during cooling. Moreover, an even short temperature undershot under the programmed IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment would unintentionally induce the start of CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization. This combination of fast cooling rates and fast equilibration at the target temperature without undershot made power-compensated DSCs better suited than heat-flux DSCs for this type of measurements.
Thanks to the low thermal mass of the Arena® furnace of the DSC 214 Polyma, it is the first DSC that combines robustness and easy handling of a heat-flux DSC with fast heating and cooling possibilities of a power-compensated DSC.
Isothermal Crystallization of LDPE
The DSC 214 Polyma was used to carry out tests of IsothermalTests at controlled and constant temperature are called isothermal.isothermal CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization at different temperatures on LDPE. Proper regulation parameters were used in order to optimize the transition from a fast cooling to the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment.
The 3.04-mg sample was heated to 150°C at 20 K/min. After a 2-minute isotherm, the polymer was cooled to eight different temperatures between 101.5°C and 98.5°C, each temperature separated from each other with 0.5°C. The sample was then maintained at the target temperature until the endo of the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization reaction.
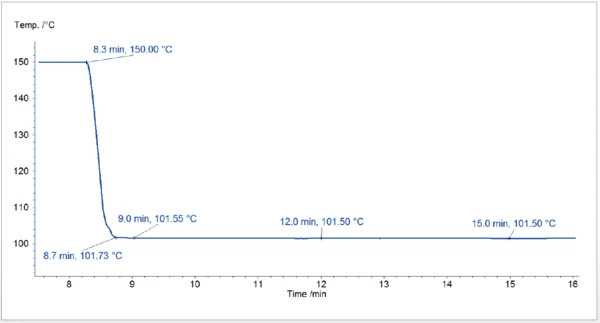
Presented in figure 1 is the temperature profile of the cooling from 150°C to 101.5°C. It shows that the target temperature is quickly achieved without undershot and that it remains stable during the complete IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment.
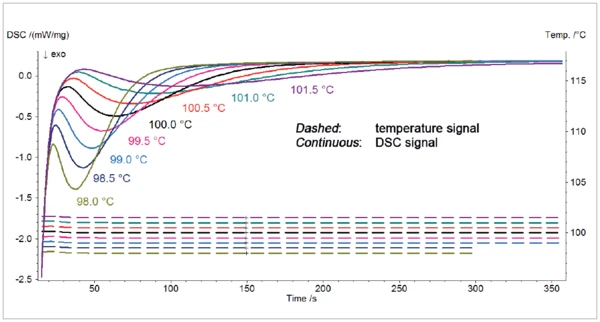
The resulting DSC cuve of the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segements at the eight temperatures of the isotherm between 101.5°C and 98.5°C are displayed in figure 2.
The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peak detected during the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment of the measurements results from theCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization of polyethylene. As expected, the reaction occurs earlier with decreasing target temperatures. The slope of the peak is higher with decreasing temperature of the isotherm. This is due to a faster reaction rate.
A difference of only 0.5°C between the temperature of the isotherm leads to big difference in the resulting DSCCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization curves, indicating a strong influence of the temperature on the reaction. An undershot, even of only some tenths of a degree, would start the reaction unvoluntarily. That is why the temperature must be well controlled during the change from cooling to the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment.
From DSC Curves to the Determination of the Activation Energy of the Crystallization Reaction
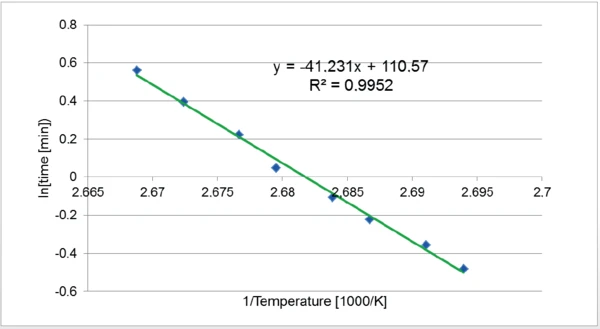
A kinetic study was carried out according to ASTM standard E2070-13 (test method - Time-to-Event), where the lapsed time at a constant conversion and at IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature T and the energy of activation E are related to the following equation:
In[Δ] = E/RT + b, where R = 8.31 J/(K∙mol)
The slope E/R of the curve In [Δt]=f(1/T) can be used to determine the activation energy of the reaction.
The time elapsed between the beginning of the isotherm and the peak maximum was determined for each temperature. Each point was plotted in the graph In(time) as a function of 1/T. The slope of the trend line allows for the determination of the activation energy of the reaction. Here, it amounted to 434 kJ/mol.