Introduction
In the age of energy transition, lightweight construction plays a central role in the automotive sector, aviation and transportation. With regard to electromobility, a weight reduction of 100 kg can save up to 0.64 KW/h per 100 km for a passenger car [1]. Due to their high specific strength, aluminum alloys count among the most important material groups in structural lightweight design. Weight savings of up to 30% can be achieved by substituting steel components with aluminum alloys [2].
AlMgSi alloys are aluminum materials with magnesium (0.6 to 1.2 mass-%) and silicon (0.4 to 1.3 mass-%) as main alloying elements [3]. They belong to the group of precipitation-hardening alloys and can be further strengthened – for example, after a forming process – by a specific heat treatment. A classification of the relevant heat treatment conditions can be found in DIN EN 515 [4].
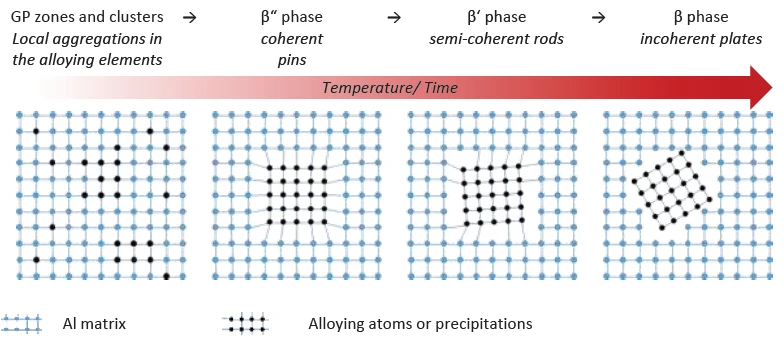
During heat treatment, finely dispersed magnesium silicide precipitates form in the material. These distort the crystal lattice of the aluminum matrix and act as an impediment to dislocation movement. The resulting strengthening effect, however, is heavily dependent on the morphology of the precipitates and their integration into the aluminum matrix (coherence). In the case of AlMgSi alloys, the following precipitation order, presented in figure 1, exists occurring with increasing temperature [5]:
The fine clusters and Guinier-Preston zones (GP zones1) that form first do not lead to any significant material strengthening. Due to the coherent needle-shaped β“ phase that develops subsequently, the alloy system reaches maximum strength. Then, the rod-shaped semicoherent β’ phase develops. This subsequently transitions into the equilibrium β phase (Mg2Si), which leads to embrittlement of the alloy due to its size (100 nm and more) and incoherence. [5]
1Guinier-Preston zones form in a metal alloy by segregation processes in which – above specific temperatures – the atoms of an alloying element assemble to form agglomerates on an atomic level up to microscopic precipitates.
Analysis of the Precipitate Morphology by Means of Differental Scanning Calorimetry
The formation and dissolution of precipitations constitute exo- or endothermal processes leading to heat absorption or release. With the aid of differential scanning calorimetry (DSC), these heats of reaction can be recorded as a function of temperature. During DSC measurements, a crucible with a sample and a reference crucible that is usually empty are subjected to a defined time-temperature program in a symmetrically designed temperature chamber. The crucible serves to avoid contamination of the measuring cell by the material being analyzed. During the experiment, both the temperature of the sample and the reference are measured by means of thermocouples. Due to the symmetrical arrangement of the sample and reference sides and a defined thermal bridge in between, the heat flow or reaction enthalpy can be determined. Thus, on the one hand, DSC allows for the determination of temperatures required for the formation of the precipitation phases; and on the other, it allows for conclusions to be drawn about the existing microstructure state based on the measured transformation enthalpies.
Metallic materials are usually characterized in high-temperature differential scanning calorimeters (above 750°C) for detection of their melting temperatures. However, depending on the material or the effect to be analyzed, low-temperature devices may also be suitable.
Due to the thermocouples – usually type E employed – low-temperature devices are characterized by a significantly higher heat-flow sensitivity in the respective measurement range than high-temperature equipment – for example, with type S thermocouples. According to DIN EN 60584-1 [7], type E features approximately eight times the thermal differential voltage per Kelvin at 300°C as a type S element. This makes low-temperature devices well suited particularly for analyzing small thermal effects.
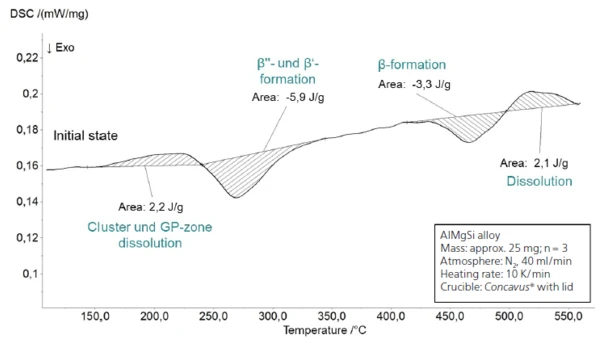
Figure 2 shows a temperature-heat flow diagram from 30°C to 450°C of an incompletely hardened AlMgSi sample, similar to a T4 state2 as used in forming operations. The measurement was carried out under an N2 atmosphere at a heating rate of 10 K/min and using Concavus® aluminum crucibles. Due to the temperature range of investigation from 30°C to 560°C and the passivation layer of the sample as well as the crucible, it can be assumed that no reaction takes place between the two. An empty crucible was selected as a reference. On the basis of a semi-finished sheet with a thickness of 1.0 mm, the samples were prepared into cylindrical disks by a process of cutting and subsequent grinding. Based on the expected relatively small transformation enthalpies of a few J/g, a comparatively large initial weight of 25 mg ± 0.5 mg was selected. For statistical safety, all measurements were carried out three times.
2 T4 state: solution-treated, quenched and naturally aged in accordance with DIN EN 515 [3]
Passivation Layer
Passivation is the formation of a kind of “protective film” on the surface of certain metals. It counteracts corrosion and is fomred by the same elemnts that trigger corrosion. The passivation layer should feature high DensityThe mass density is defined as the ratio between mass and volume. density and low porosity. At the same time, for high compatctness, the layer must be very thin and homogeneously distributed over the metal surface.
The NETZSCH low-temperatur DSC features a highly precise measuring sensor (enthalpy precision < 1% for Indium) and – depending on the cooling system used – allows for measurements to 750°C (according to model) and for heating and cooling rates between 200 and 500 K/min (according to module). It is further equipped with a gas-tight measuring cell, allowing for coupling to a Fourier Transform Infrared Spectrometer (FT-IR) or a mass spectrometer (MS) as well as the setting of defined atmospheres.
In the first endothermal effect from approx. 150°C to 240°C, small clusters and GP zones that are present in the microstructure and and act as nuclei dissolve (fig. 2). Furthermore, larger precipitates continue to grow. Above a critical nucleation size, an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic reaction occurs from approx. 240°C to 340°C; this is attributable to the formation of the coherent β‘ and semicoherent β“ phase. Direct differentiation of the caloric signals cannot be carried out on the basis of the measurement. Both Fang et al. [8] and Gaber et al. [6] document an overlapping of the two precipitation peaks dependent on the ratio between Mg and Si, which also prevents separation of the caloric effects there. The exact composition of the alloys investigated here is not known, so further conclusions cannot be drawn. Beginning at approx. 410°C, the incoherent β phase is formed. Directly after this (beginning at approx. 500°C), these precipitates dissolve again, which explains the last endothermal effect.
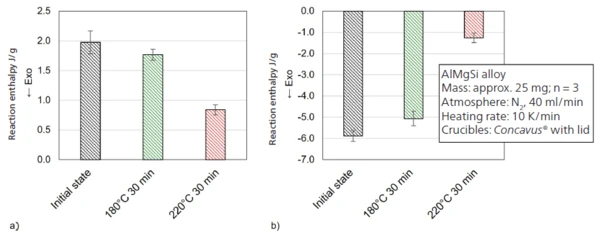
Presented in figure 3 is the influence of a preceding half-hour heat treatment at 180°C as well as 220°C in comparison to the initial state. The heat treatment was realized in the DSC – in a previous program section not displayed here. The diagram shows subsequent heating to 560°C. Treatment for 30 minutes at 180°C tends to reduce the endothermal peak at approx. 220°C. Compared to the initial state, the average enthalpy decreases from 1.98 ± 0.19 J/g to 1.77 ± 0.09 J/g (figure 4 a). Furthermore, the peak area of the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal precipitation of the β‘ und β“ phase at approx. 270°C also decreases slightly from -5.88 ± 0.26 J/g to -5.07 ± 0.34 J/g (figure 4 b). It can be assumed that both reactions, i.e., the dissolution of subcritical cluster and GP zones along with the formation of the β‘ or β“ phase, have taken place to a minor extent during the preceding heat treatment at 180°C.
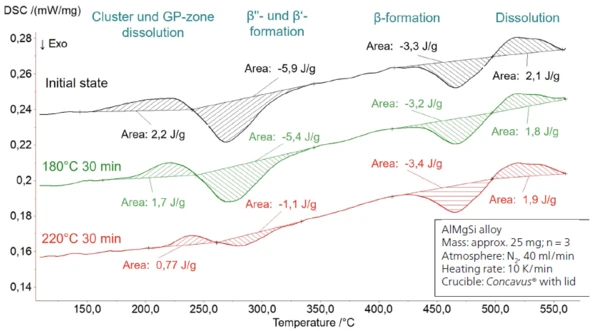
Increasing the temperature to 220°C with the same hold time enlarges the effect. As shown in figures 4a) and 4b), both the endothermal dissolution peak and the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal precipitate formation are significantly reduced, to values of 0.84 ± 0.09 J/g and -1.26 ± 0.22 J/g, respectively. In conclusion, a large proportion of β‘ or β“ phases are already present in the microstructure. The extent to which the remaining precipitation potential contributes to the material’s increase in strength, or the extent to which the temperature program can be optimized, should be determined using also mechanical testing such as tensile tests. An important detail is that in the case of both temperature treatments, the reaction enthalpy of the growth of the β phase (ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effect at approx. 410°C) and the subsequent endothermal dissolution of the precipitates do not change substantially (see figure 3).
Summary
AlMgSi alloys are aluminum materials which can be strengthened by temperature-induced precipitate formation. The formation and dissolution of the finely dispersed magnesium silicide precipitates thereby constitute exo- and endothermal effects in the one-digit J/g range. Low-temperature differential calorimeters are usually employed for the analysis of low-melting substances, such as polymers, and particularly feature high heat-flow sensitivity. With the aid of low-temperature DSC, these effects can precisely be quantified. Based on comparative measurements, conclusions on the formation temperatures and the resulting morphology can be drawn. Along with the fundamental analysis of the mechanisms taking place, both energetic- and strength-optimized heat treatment layouts can be designed in combination with other test methods, such as uniaxial tensile tests.