Introduction
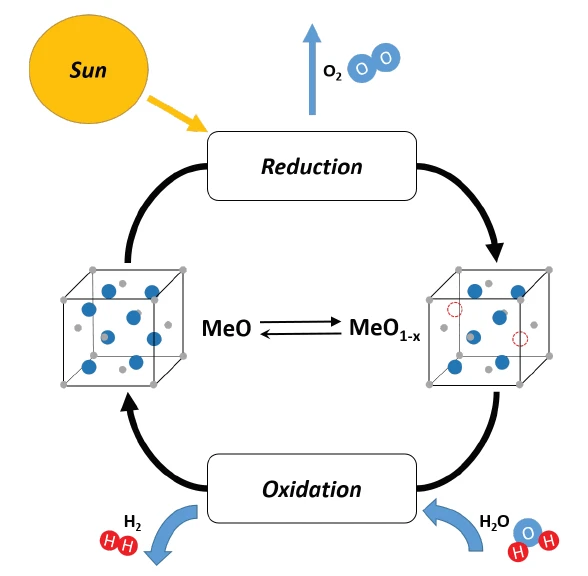
Thermochemical water splitting is a process used for hydrogen production employing high-temperature heat (500°C to 2000°C) and a series of chemical reactions. The chemicals used in the process are reused in each cycle, creating a closed loop that consumes only water and produces hydrogen and oxygen. Thus, thermochemical hydrogen production is an environmentally friendly alternative to fossil-fuel-based hydrogen production systems [1].
Measurement Conditions
To investigate thermochemical water splitting on LSC20 (La0.8Sr0.2CoO3), thermogravimetric measurements (TGA) were performed using the NETZSCH STA 449 F3 Jupiter®. For the supporting interpretation, the thermal analyzer was additionally coupled with the NETZSCH QMS Aëolos® Quadro quadrupole mass spectrometer. A detailled compilation of the exact measurement conditions can be found in table 1.
Table 1: Measurement parameters
| Parameter | Thermochemical Water Splitting on LSC20 |
|---|---|
| Device | STA 449 F3 Jupiter® |
| Accessories | Water-vapor furnace and vapor generator |
| Sample Carrier | TGA, type S |
| Crucible | TGA plate made of Al2O3 with a diameter of 17 mm |
| Sample Weight | 215.46 mg powdered sample) |
| Measurement program | RT to 1200°C, 15 K/min, 4% H2 in argon 90 min isotherm @ 1200°C, 4% H2 in argon 1200 °C to 600°C, 15 K/min, 4% H2 in argon 30 min isotherm @ 600°C, argon 60 min isotherm @ 600°C, 33% H2O in argon 30 min isotherm @ 600 °C, argon |
Results and Discussion
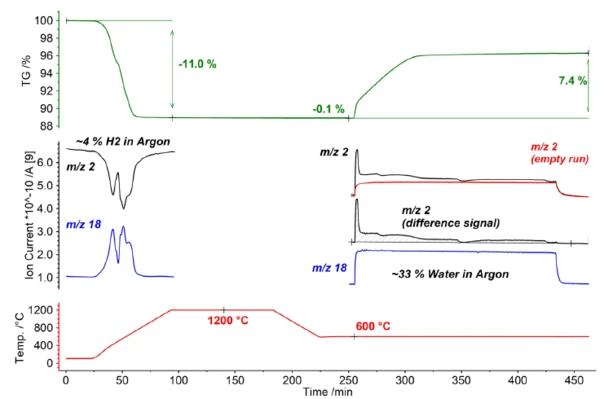
In the first step of the investigation, LSC20 was activated using a reducing atmosphere (4% H2 in argon). Thereby, the sample material shows a pronounced mass loss of -11.0%. Furthermore, the consumption of hydrogen (mass number 2) with the simultaneous release of water (mass number 18) can be clearly observed by means of the simultaneously coupled mass spectrometer (see blue and black curves in figure 2).
The actual thermochemical water splitting takes place in the second part of the investigation. To this end, the sample was cooled to 600°C and then exposed to a gas atmosphere containing water (33% H2O in argon). This resulted in an oxidatively induced mass increase of 7.4% with the simultaneous release of hydrogen (see mass number 2 in figure 2). Based on the abrupt changes in the mass curve as well as the Ionic current curve of the mass spectrometer, it can be seen that water splitting is a multistage process; this suggests a direct surface reaction as the initial reaction step as well as a diffusion-controlled reaction in the further course.
Summary
The platform concept of the NETZSCH STA 449 F3 Jupiter® provides an excellent basis for replicating intricate thermal processes and phenomena. In the presented example, a targeted investigation of a thermochemical water splitting reaction was successfully reproduced using a custom-designed water vapor furnace and a steam generator.
Not only were the weight changes accurately measured (gravimetric recording) in this example, but the processes occurring during the reaction were also analyzed and interpreted. This was achieved by employing coupled mass spectrometry to examine the gases released during the reaction.
The combination of these instruments – STA, water vapor furnace, steam generator, and coupled mass spectrometer – creates an ideal setup for comprehensively characterizing the ongoing reactions involved in thermochemical water splitting.