Introduction
Photo Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing of liquid monomers and oligomers is employed in a variety of industries as an environmentally friendly, safe, fast, and easily controlled approach to forming inks, coatings, adhesives, and structural materials. The expansion of applications for photo Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing since its introduction in the 1960s has been accompanied by an evolution in the light sources used. For example, stereolithography, an additive process for manufacturing 3-dimensional objects from photo-curable polymer resin, requires a laser to trace complex patterns on each layer of liquid resin.
The ability to measure Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing kinetics and Degree of CureThe degree of curing describes the conversion achieved during crosslinking reactions (curing). degree of cure is essential to the selection of suitable UV and visible light sources, the identification of optimal Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing times and conditions, and the development of new, photo curable resins. Photo-differential scanning calorimetry (Photo-DSC) and photo-dielectric analysis (Photo- DEA) are powerful analytical tools for accomplishing these measurements.
In the example presented here, the efficiencies of two different UV light sources were compared in the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing of a water-soluble, blue-Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing adhesive. Laser Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing was employed, for the first time, in combination with DSC and DEA measurements and compared with the standard mercury (Hg) arc lamp. The pre-polymer formulation consisted of polyethylene glycol diacrylate (PEGDA) with camphorquinone (CQ) photo-initiator (1% by weight relative to PEGDA) and N,N-dimethyl-p-toluidine (DMPT) as a coinitiator (1:1 by weight relative CQ). This formulation has been used to fabricate complex hydrogel scaffolds with a fully interconnected pore network for use as bioreactors1.
1Paul Calvert, Swati MIshra, Amrut Sadacher, Dapeng LI, University of Massachusetts, Dartmouth, NTC Project: F06-MD14, National Textile Center Research Briefs: June 2010
Photo-DSC Measurements
DSC measurements were performed using a NETZSCH DSC 204 F1 Phoenix® interfaced with either an OmniCure® S2000 200 watt Hg short-arc lamp (Figure 1) with a band pass filter delivering a spectral range of 320-500 nm with an irridiance of 10 W/cm² or a LASERGLO W Technologies LRD-0447 Series collimated diode laser system (Figure 2) delivering 447 nm wavelength of 0.744 W/cm2.


Figure 3 and Figure 4 show the results of three sets of DSC measurements of the resin Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing under multiple 2-second pulses from the Hg arc lamp and from the laser, respectively. Degree of CureThe degree of curing describes the conversion achieved during crosslinking reactions (curing). Degree of cure calculations based on peak areas from the three lamp runs and the three laser runs are listed in Table 1 and Table 2, respectively. The measurements exhibited good reproducibility.
The total resin Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing enthalpy was greater for the laser (129±5 J/g) than for the lamp (91±6 J/g).2 The corrected enthalpy of each peak from the laser runs was, on average, greater than the corresponding peak from the measurements with the lamp. Furthermore, unlike the lamp, the laser continued to generate additional Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing enthalpy up to the final pulse in the measurement. The residual peak area at the end of the cure (e.g. pulse No. 15) is attributable to the heating effect of the light source on the sample, which was nine times greater for the lamp than for the laser.
2Total curing enthalpy was calculated by totaling the peak areas and subtracting out the baseline contribution from differential heating of the sample and reference crucibles, which was calculated from the enthalpy of the final pulse in the series. The timing of the Omnicure lamp pulses was controlled by the NETZSCH Proteus® software. The timing of the laser pulses was controlled manually.
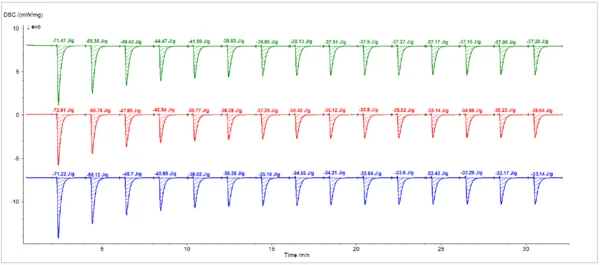
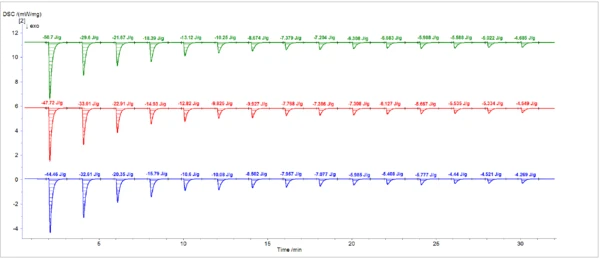
Table 1: Degree of CureThe degree of curing describes the conversion achieved during crosslinking reactions (curing). Degree of cure calculations (Hg lamp)
First Run | Second Run | Third Run | |||||||
|---|---|---|---|---|---|---|---|---|---|
Pulse No. | Peak area (Jg) | Corrected enthalpy J/g) | Conversion (%) | Peak area (Jg) | Corrected enthalpy (J/g) | Conversion (%) | Peak area (J/g) | Corrected enthalpy (J/g) | Conversion (%) |
| 1 | 71.47 | 34.19 | 40.51 | 72.91 | 37.87 | 40.29 | 71.22 | 38.08 | 40.24 |
| 2 | 58.35 | 21.07 | 34.96 | 56.78 | 21.74 | 23.13 | 55.12 | 21.98 | 23.23 |
| 3 | 49.42 | 12.14 | 14.38 | 47.85 | 12.81 | 13.63 | 45.7 | 12.56 | 23.23 |
| 4 | 44.47 | 7.19 | 8.52 | 42.54 | 7.50 | 7.98 | 40.88 | 7.74 | 8.18 |
| 5 | 41.59 | 4.31 | 5.11 | 39.77 | 4.73 | 5.03 | 38.02 | 4.88 | 5.16 |
| 6 | 39.93 | 2.65 | 3.14 | 38.28 | 3.24 | 3.45 | 36.38 | 3.24 | 3.42 |
| 7 | 38.86 | 1.58 | 1.87 | 37.25 | 2.21 | 2.35 | 35.18 | 2.04 | 2.16 |
| 8 | 38.13 | 0.85 | 1.01 | 36.42 | 1.38 | 1.47 | 34.55 | 1.41 | 1.49 |
| 9 | 37.91 | 0.63 | 0.75 | 36.12 | 1.08 | 1.15 | 32.21 | 1.07 | 1.13 |
| 10 | 37.50 | 0.22 | 0.26 | 35.80 | 0.76 | 0.81 | 33.84 | 0.70 | 0.74 |
| 11 | 37.27 | -0.01 | -0.01 | 35.52 | 0.48 | 0.51 | 33.60 | 0.46 | 0.49 |
| 12 | 37.17 | -0.11 | -0.13 | 35.14 | 0.10 | 0.11 | 33.43 | 0.29 | 0.31 |
| 13 | 37.06 | -0.12 | -0.14 | 34.95 | -0.09 | -0.10 | 33.29 | 0.15 | 0.16 |
| 14 | 37.09 | -0.19 | -0.23 | 35.23 | 0.19 | 0.20 | 33.17 | 0.03 | 0.03 |
| 15 | 37.28 | 0.00 | 0.00 | 35.04 | 0.00 | 0.00 | 33.14 | 0.00 | 0.00 |
Total enthalpy = 84.40 J/g | Total enthalpy = 94.00 J/g | Total enthalpy = 94.63 J/g | |||||||
Table 2: Degree of CureThe degree of curing describes the conversion achieved during crosslinking reactions (curing). Degree of cure calculations (laser)
First Run | Second Run | Third Run | |||||||
|---|---|---|---|---|---|---|---|---|---|
Pulse No. | Peak area (Jg) | Corrected enthalpy J/g) | Conversion (%) | Peak area (Jg) | Corrected enthalpy (J/g) | Conversion (%) | Peak area (J/g) | Corrected enthalpy (J/g) | Conversion (%) |
| 1 | 50.70 | 46.02 | 35.40 | 47.72 | 43.17 | 32.56 | 44.46 | 40.19 | 32.47 |
| 2 | 29.60 | 24.92 | 19.17 | 33.01 | 28.46 | 21.47 | 32.61 | 28.34 | 22.89 |
| 3 | 21.67 | 16.99 | 13.09 | 22.91 | 18.36 | 13.85 | 20.35 | 16.08 | 12.99 |
| 4 | 18.39 | 13.71 | 10.54 | 14.93 | 10.38 | 7.83 | 15.79 | 11.52 | 9.31 |
| 5 | 13.12 | 8.44 | 6.49 | 12.82 | 8.27 | 6.24 | 10.6 | 6.33 | 5.11 |
| 6 | 10.25 | 5.57 | 4.28 | 9.83 | 5.28 | 3.98 | 10.09 | 5.81 | 4.69 |
| 7 | 8.67 | 3.99 | 3.08 | 9.93 | 5.38 | 4.06 | 8.502 | 4.23 | 3.42 |
| 8 | 7.38 | 2.69 | 2.07 | 7.77 | 3.22 | 2.43 | 7.957 | 3.69 | 2.98 |
| 9 | 7.20 | 2.52 | 1.94 | 7.39 | 2.84 | 2.14 | 7.077 | 2.81 | 2.27 |
| 10 | 6.31 | 1.62 | 1.25 | 7.31 | 2.76 | 2.08 | 5.985 | 1.72 | 1.39 |
| 11 | 5.68 | 1.00 | 0.77 | 6.13 | 1.58 | 1.19 | 5.408 | 1.14 | 0.92 |
| 12 | 5.99 | 1.30 | 1.00 | 5.67 | 1.12 | 0.84 | 5.777 | 1.51 | 1.22 |
| 13 | 5.59 | 0.90 | 0.69 | 5.54 | 0.99 | 0.74 | 4.44 | 0.17 | 0.14 |
| 14 | 5.02 | 0.34 | 0.26 | 5.33 | 0.78 | 0.59 | 4.521 | 0.25 | 0.20 |
| 15 | 4.69 | 0.00 | 0.00 | 4.55 | 0.00 | 0.00 | 4.269 | 0.00 | 0.00 |
Total enthalpy = 128.99 J/g | Total enthalpy = 132.58 J/g | Total enthalpy = 123.79 J/g | |||||||
Photo-DEA Measurements
DEA monitoring of the resin photocuring process at ambient temperature using the two different light sources were performed with a NETZSCH DEA 288 Epsilon instrument (Figure 5). The results are compared in Figure 6. Two measurements were performed with each radiation source in order to demonstrate reproducibility. Both the laser and lamp were run continuously with the exception of a two minute interruption in irradiation from the lamp during one of the runs. Curing progress is indicated by an increase in Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.Ion Viscosity, which levels off as curing completes. The initial slopes of the Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.Ion Viscosity curves are slightly greater for laser-cured samples than for the lamp-cured ones, indicating more efficient curing from the laser. The overall increase in the Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.Ion Viscosity was also slightly greater for the laser-cured samples. DEA measurements are more sensitive to small changes in the Degree of CureThe degree of curing describes the conversion achieved during crosslinking reactions (curing). degree of cure than DSC measurements. Hence, increases in the Ion Viscosities of the samples due to curing were still measurable after 50 minutes of continuous lamp or laser irradiation. Due to sample heating by the lamp or laser, which causes an increase in ion mobility, sharp steps in the curves are observed as soon as the light source is removed.

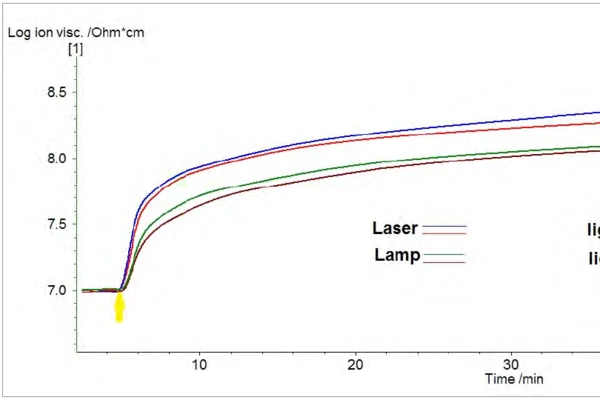
Summary
In summary, a comparison of the curing enthalpy and curing kinetics of photo-curable resin under irradiation with a Hg arc lamp and a blue diode laser was accomplished using NETZSCH photo-DSC and photo-DEA instrument configurations. DSC measurements showed that the enthalpy of resin curing with the laser was greater than that with the lamp, indicating possibly greater crosslinking of the sample with the laser. This is consistent with the greater absolute change in the Ion ViscosityIon viscosity is the reciprocal value of the ion conductivity, which is calculated from the dielectric loss factor.Ion Viscosity of the laser cured sample measured by DEA. The DEA measurements also showed that the resin curing rate was slightly greater with the laser than with the lamp. Finally, the DSC measurements indicated greater sample heating by the Hg lamp radiation than by the laser radiation. Sample heating can be an issue in cases where temperature changes during the polymerization lead to polymer shrinkage StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress. Overall, the lower intensity, monochromatic blue laser proved to be a more suitable light source for curing this particular resin formulation than the Hg arc lamp with a broad-band filter.