Introduction
The purity of pharmaceutical and food products goes hand in hand with their quality. Delivered substances should not contain any contaminants that could be harmful to an organism. Particularly in formulations, contaminants should not interfere with the active ingredient and thus hinder its proper functioning. For these reasons, purity determination is essential for drug, cosmetic and food substances.
In the present example, the purity of Nipagin was determined. This white powder is known by the chemical name of methyl paraben (figure 1) and is used as a preservative in cosmetics, medications, and in foods under the name E218 [1, 2].
ASTM E928-08 describes the procedure for purity determination by means of DSC measurements. It takes into account that “the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature range of a compound broadens as the impurity level rises” [3]. This method is applicable for mixtures with impurities which dissolve in the melt and are insoluble in the crystal, thus for what are known as eutectic impurities.
Test Conditions
The Importance of Thermal Resistance – Time-Saving Software Solution by NETZSCH
Temperature changes during a DSC measurement are usually measured on the reference side. The “true” sample temperature depends on the thermal resistance between the reference and sample crucibles as well as on the enthalpy of the processes occurring in the sample. Because knowledge of the correct sample temperature plays an important role in the determination of purity, the thermal resistance has to be calculated prior to the tests. In the NETZSCH Proteus® software for DSC, the calibration regarding the thermal resistance takes place simultaneously with temperature and enthalpy calibration, so that the resulting curves automatically show the true temperature inside the sample.
Measurements
Prior to the measurements with the DSC 204 F1 Phoenix®, the Concavus® pans made of aluminum were washed in acetone and heated to 425°C for one minute. After placing the sample (sample mass 2.12 mg) in the crucible, it was hermetically sealed and positioned in the DSC cell.
The temperature range has to be carefully chosen in order to start heating well prior to melting because the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point can be depressed by the influence of impurities. Furthermore, pre-melting has to be considered, which is minimal in very pure materials but can increase with increasing contamination.
In the first segment, the sample was heated from room temperature to 100°C at a heating rate of 20 K/min. In the following segment, the heating rate was decreased to 0.7 K/min and the temperature was increased to 130°C. During the entire experiment, the DSC cell was purged with dry nitrogen.
Test Results
Figure 2 shows the DSC curve of the 2nd heating segment. The endothermal peak results from the melting of Nipagin. The detected onset temperature at 125.4°C is in good agreement with the literature value for the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature (125.2°C [1]).
For the purity calculation, the Van't Hoff equation is used, as described in method A of ASTM E928-08:
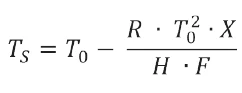
TS: sample temperature [K]
T0: Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state.
The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of pure Nipagin [K]
R: gas constant (= 8.314 J/mol-1·K-1)
X: mol fraction of impurity
H: heat of fusion [J·mol-1], calculated from the peak area
F: fraction melted
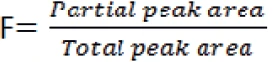
The Van't Hoff plot is a graphical display of the data from the purity determination. It compares the actual measurement data for 1/F (the reciprocal of the fraction of the melting peak) with the temperature at which that amount of melting is observed. This data is usually nonlinear; non-linearity increases with decreasing purity. The deviation from linearity is caused by pre-melting, which cannot be detected by means of DSC. One should note that the curvature is influenced by the temperature program of the DSC measurement (starting too close to the melting peak) and the limits for the calculation of the peak area (e.g., left limit too close to the melting peak).
For linearization of the curve, the software calculates a revised value for F by adding a correction factor c to the total area and to each fractional area. This procedure finally achieves the linearity of the curve Ts=f(1/F).
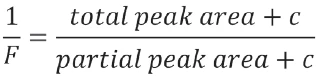
In addition, the molecular weight must be entered in the software to calculate the mole% value.
Figure 3 exhibits the observed and the corrected data (linear curve).
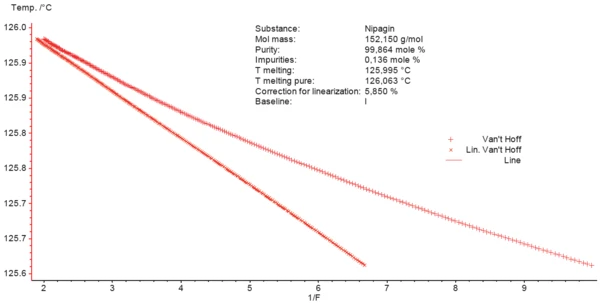
Purity is calculated from the slope of the corrected linear data. The theoretical Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of 100% pure material can also be obtained from the plot as the point where the fraction melted (1/F) is 0 (T melting pure in the box of Figure 3). It amounts to 126.063°C compared to the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of 125.995°C. The NETZSCH Purity software computes the impurity content of the measured Nipagin sample to be 0.14 mole-%.
The results are only reliable when the adjusted data shows linearity, the purity level is higher than 98.5% and the correction factor c is lower than 20% [3].
After the measurement, the sample was weighed again to ensure that no mass loss occurred during the measurement. A change in the initial mass would indicate evaporation of volatiles, which would result in an endothermal effect. Thus, the EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic peak would not only be caused by melting, but also by the release of volatiles. This would distort the peak evaluation.
Conclusion
The DSC method provides an easy way to determine the purity of pure crystalline materials. The purity is calculated using the rate of melting of the material under investigation. From the DSC melting peak, the depression of the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point is determined, which is directly related to the presence of impurities.
A prerequisite for purity determination by means of DSC is that the impurities dissolve in the melt and are insoluble in the crystal. For correct purity determination, sublimation of the sample should be also prevented.