Introduction
When water comes into contact with a crystalline substance, various types of interactions are possible: The water molecules may simply adsorb on the surface; condensed liquid water may appear on the solid (in the case of deliquescence or capillary condensation); or, the water may even be incorporated into the crystal structure (absorption), forming stoichiometric or non-stoichiometric hydrates [1]. During heating, in turn, different amounts of energy are required to overcome these interactions and break the bonds formed. That’s why we sometimes see several mass-loss steps when a hydrate is heated; water molecules adsorbed on the surface desorb first, followed by water which is bound more strongly.
For the design of a dehydration process, it is therefore very important to know the thermal properties of the particular sample. Thermogravimetric analysis in combination with kinetic evaluation is extremely useful here because it can help significantly reduce the time it takes to develop a suitable temperature program. If the thermal measurements are performed by means of hyphenated systems, e.g., by means of TGA or STA coupled to a gas analyzing system such as FT-IR, then it is additionally feasible to find out whether the gas evolved during heating is really just water or whether further volatiles are involved.
Magnesiumstearat as a Model Substance –Experimental
Magnesium stearate is one of the most widely used excipients in the pharmaceutical field. It is typically used as a lubricant added to solid dosage forms such as tablets. Many commercial magnesium stearate types are composed of a mixture of various hydrates: monohydrate (ordered or disordered), dihydrate and/or trihydrate. [2] For the present series of experiments, approx. 6.5 mg of magnesium stearate powder, as received, was heated at heating rates between 2 K/min and 20 K/min using a NETZSCH TG 209 F1 device. The complete set of measurement parameters is given in table 1.
Table 1: Measurement parameters
| Parameters | Magnesium Stearate |
|---|---|
| Sample mass | Approx. 6.5 mg |
| Atmosphere | Nitrogen |
| Crucible | Al, open |
| Temperature program | RT to 180°C |
| Heating rates | 2, 5, 10 and 20 K/min |
| Flow rate | 40 ml/min |
| Sample holder | TGA, type P |
Results and Discussion
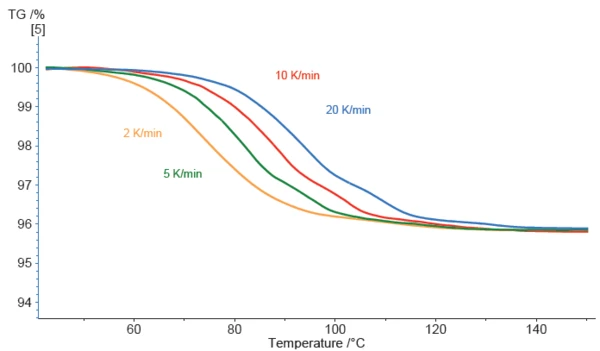
The observed mass loss of the magnesium stearate sample starts quite early. In the curve performed at 2 K/min, a deviation is already visible at about 50°C.
The higher the applied heating rate, the more the curves are shifted to higher temperatures which is characteristic for kinetic effects. Furthermore, the curves at higher heating rates exhibit more evident structure. In the blue curve (carried out at 20 K/min), three mass-loss steps can clearly be detected. This indicates that reducing the heating rate does not always improve separation of overlapping effects – sometimes the opposite is true, as in the present example. Thus, the kinetics behind the mass-loss effects are crucial.
In order to learn more about the kinetics behind the mass-loss effects, the NETZSCH Kinetics Neo software was subsequently applied. Using the software, it was possible to fit the experimental data well by applying a three-step consecutive model of nth order reactions (t:FnFnFn, see fig. 2)
A → B → C → D
The corresponding correlation coefficient R2, which is an indicator of the fit quality, was determined to be 0.99993.
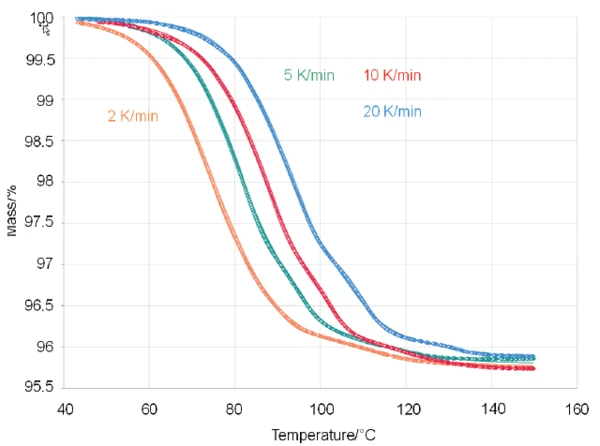
Kinetics Neo is a formal kinetics software which can analyze different kinds of temperature-dependent chemical processes, whether they are associated – amongst ther possibilities – with a mass change, length change or enthalpy change. Kinetics Neo can work on the basis of model-free and model-based methods. The model-based kinetic approach is capable of providing information about each reaction step along with related parameters such as activation energy, reaction order or the contribution to the total process. The calculated parameters for the present case are listed in table 2.
Based on these findings, predictions can be calculated for temperature profiles which were not previously measured, or which are not even accessible experimentally at all.
This was done for the following two scenarios:
1. The first one is a simulation of the classical loss on drying procedure in a drying chamber which is set to 105°C. [3], [4]
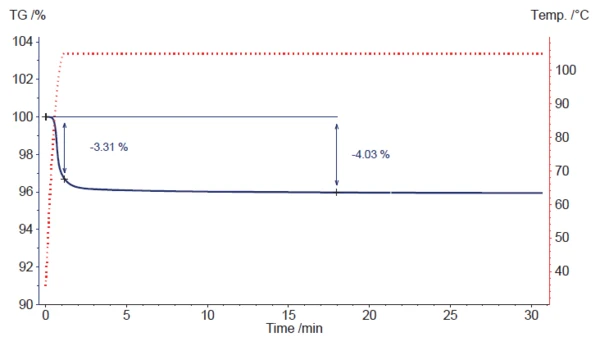
To simulate the direct insertion of the sample into a hot drying chamber, 100 K/min was selected as the initial heating rate, followed by an IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment at 105°C (see fig. 3).
Mass loss begins during the fast heating phase but cannot finish completely. Only about 3.3% of the mass loss occurs before the switchover to the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment takes place. After approx. 18 minutes, a mass loss of 4.03% is reached, which agrees well with the value of 4.02% given on the analysis certificate for the magnesium stearate used.
Table 2: Formal kinetic parameters of the dehydration process of magnesium stearate
| Parameters | A → B Fn | B →C Fn | C →D Fn |
|---|---|---|---|
| Activation energy [kJ/mol] | 122.34 | 129.25 | 217.42 |
| Log pre-exponential factor | 16.15 | 16.46 | 27.59 |
| Reaction order | 0.853 | 0.948 | 3.007 |
| Contribution | 0.553 | 0.349 | 0.009 |
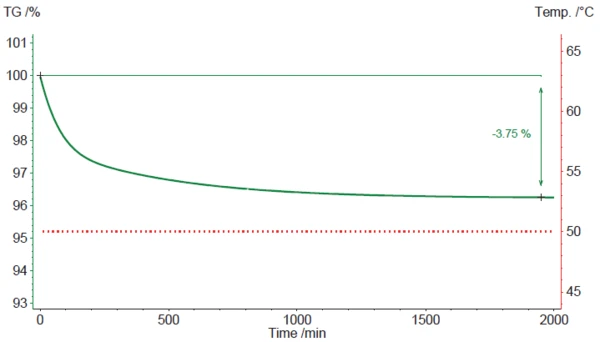
2. The second scenario is a simulation of an IsothermalTests at controlled and constant temperature are called isothermal.isothermal treatment of the magnesium stearate sample at 50°C (fig. 4).
In this case, the observed mass loss starts immediately and drags on for a long time. After approx. 32 hours (1920 minutes), it amounts to 3.75%. Just 0.27% (based on a mass loss reference value of 4.02%; see above) is left. This value is more or less maintained even if the time is extended to 80 or 160 hours. This suggests that magnesium stearate tends to lose most – but not all – of its (hydrate) water if it is stored under dry and hot conditions for a longer time. For complete dehydration, however, a temperature of 50°C seems not to be sufficient.
Conclusion
Kinetic evaluation through the application of NETZSCH Kinetics Neo offers the opportunity to determine a mathematical model which describes the experimental behavior of samples during thermal treatment. Although it is a formal description for technical purposes and usually does not reflect the full chemical mechanism behind the process, it can provide valuable clues as to what is going on in the sample. With regard to dehydration processes, this allows us to easily determine which temperature profile seems to be more promising – and all this without a laborious trial and error approach.