Introduction
In pharmacy, there is hardly any active ingredient about which more has been written than acetylsalicylic acid (or ASA for short; in English-speaking countries even the brand name Aspirin™ is often used as a synonym). Its success story began at the end of the 19th century when Dr. Felix Hoffmann synthesized the substance at the BAYER laboratories for the first time without impurities. Nowadays, it is still one of the most popular pharmaceuticals used across a broad therapeutic range. It belongs to the group of non-steroidal anti-inflammatory drugs (NSAIDs) and is indicated for the treatment of pain, fever and inflammation. In addition, it is used to prevent recurrence of heart attack or stroke in high-risk patients. In 1977, ASA was added as an analgesic to the “essential drug list” of the WHO (World Health Organization) [1].
This is the second of four application notes that examine in more detail the thermal behavior of acetylsalicylic acid: Decomposition in different gas atmospheres, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition kinetics, and the resulting gas species [2] [3] [4].
Kinetic Analysis of Thermoanalytical Data
With the measurement data of thermoanalytical methods, information about the mass loss due to Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition, PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis or combustion, about energetic changes such as melting or CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization or also about changes in sample dimension due to thermal expansion or SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering, for example, in ceramic materials, can be obtained. With these statements, however, the information content is not being used exhaustively. With the aid of a more comprehensive kinetic analysis, it is also possible to obtain information about the timely course of a reaction at different temperatures, i.e., the reaction rate. If the course of a reaction can be described sufficiently well with the help of a system of mathematical equations, it is also possible to make predictions on the reaction course that are experimentally not accessible or only with difficulty. This, in turn, can be used to optimize processes or to predict the service life, oxidative stability or aging behavior of materials and products.
Results and Discussion
In order to better understand the thermal behavior of acetylsalicylic acid, a kinetic approach was carried out in an attempt to find a system of mathematical equations for the description of experimental data. The thermal behavior was studied using a NETZSCH TG 209 F1 Libra® and applying the measurement conditions summarized in table 1. A kinetic approach requires a series of at least three different heating rates in order to describe the time-temperature correlation, which is the main objective of kinetic evaluations in general.
Table 1: TGA measurement parameters
| Parameters | Acetylsalicylic Acid |
|---|---|
| Sample mass [mg] | 4.982 │ 5.014 │ 5.053 |
| Atmosphere | Argon |
| Crucible | Al2O3, 85 μl, open |
| Temperature program | RT - 450°C |
| Heating rates [K/min] | 3 │ 10 │ 30 |
| Gas flow rate [ml/min] | 40 |
| Sample holder | TGA |
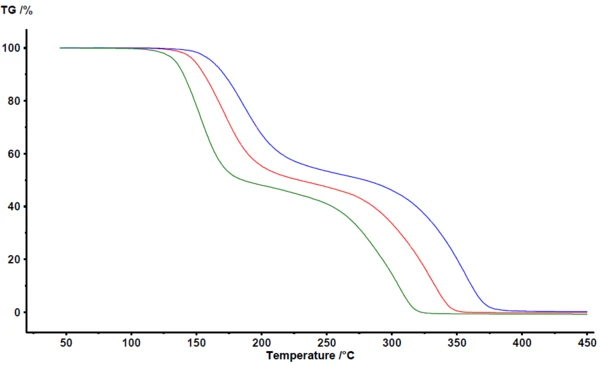
Figure 1 depicts the results as obtained in the NETZSCH Proteus® analysis software. Between 100°C and 400°C, thermogravimetry detects two major mass-loss steps for the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of acetylsalicylic acid. The TGA curves are shifted to higher temperatures with increasing heating rate. The largely parallel shift, as well as the nearly identical final mass, indicate that the heating rate itself does not significantly change the reaction mechanism. This is also a clear indication that the reaction mechanism is not very complex in this case. On the other hand, it can be clearly seen that the mass-loss steps are not perfectly separated. There is no plateau visible clearly defining the end of the first mass-loss step or the beginning of the second mass-loss step. As confirmed by coupling techniques such as TGA-FT-IR, TGA-MS or TGA-GC-MS, both PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis and evaporation occur simultaneously [2][4][5].
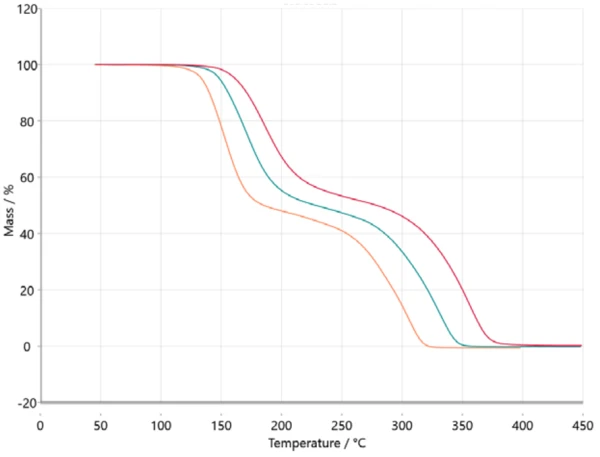
For kinetic analysis, measured data is transferred in the NETZSCH Kinetics Neo software via ASCII. Imported data is depicted in figure 2.
In order to get an initial idea about the reaction mechanism, it is useful to start the kinetic analysis with so-called model-free approaches. Figure 3 shows the results in accordance with Ozawa-Flynn-Wall, where the logarithm of the heating rate is plotted versus inverse temperature. This approach not only considers all measured data points but also delivers information about the change in the activation energy as well as the pre-exponential factor over the entire course of the reaction (degree of conversion). This is of particular help for multi-step reactions. The plot describes the progression of the reaction (from right to left) for all three heating rates (horizontal symbols). The almost vertical lines connect the same degree of conversion for each heating rate and are therefore called iso-conversional lines.
These iso-conversional lines are more or less parallel at the ranges for the two main PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis steps at the beginning and at the end of the entire process. At about 50% conversion, the iso-conversional lines show a different slope that indicates a change in reaction mechanism. At that stage of the reaction, PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis and evaporation occur simultaneously as mentioned earlier [2][4][5].
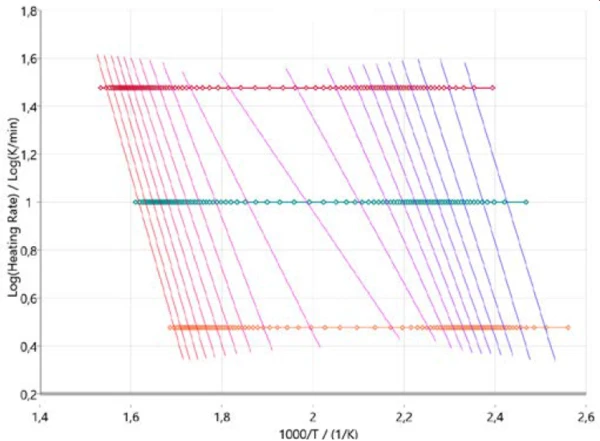
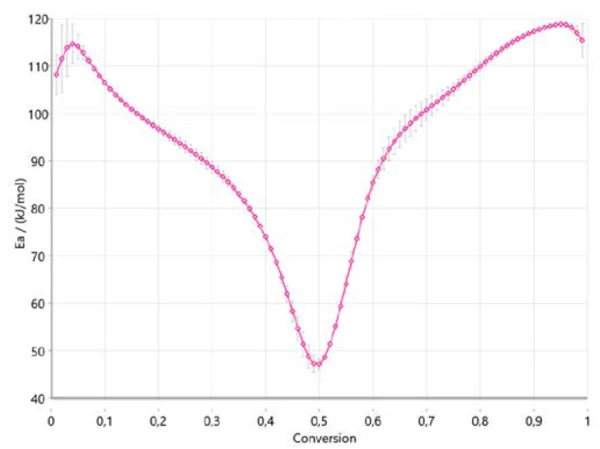
Figure 4 shows how the activation energy changes with the reaction’s progress in accordance with Ozawa-Flynn-Wall. This is very important information since it already indicates three ranges for the entire process, with an activation energy around 110 kJ/mol at the beginning, around 40 kJ/mol between 40% and 50% conversion, and around 120 kJ/mol at the end of the reaction. A change in activation energy with the degree of conversion confirms a multi-step reaction mechanism. The values obtained are in good correlation with the results published in literature [6].
Transferring this information into a model-based analysis leads to a three-step consecutive model (t:FnFnFn), where A represents the starting material (acetylsalicylic acid), B and C are intermediates known from literature [6, 7] and D is the final product. In this case, the final product is, of course, not really a substance but it describes the end of the reaction or 100% conversion since the residual mass for all three thermogravimetric curves is zero. All products formed are gaseous and therefore evaporated out of the crucible while heating to the final temperature. Figure 5 depicts the result of this model-based approach. The measured data is presented as symbols and the results for the calculated three-step consecutive model are presented as solid lines with the colors related to the different heating rates. The calculated model fits almost perfectly to the experimental data, which is ultimately confirmed by the correlation coefficient of 0.99986.
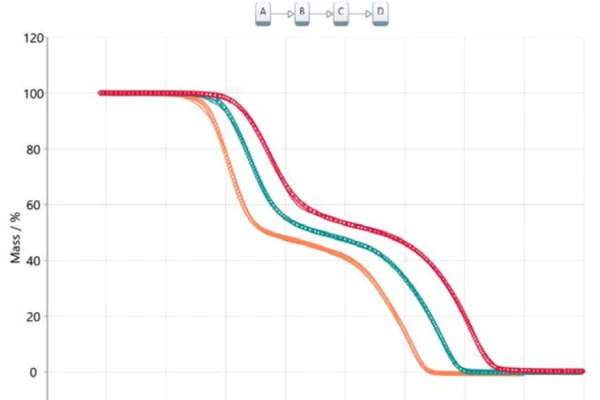
The calculated parameters pre-exponential factor, activation energy and reaction order are summarized in table 2 for each individual reaction step. All values for the activation energy are in good agreement with the values suggested by the Ozawa-Flynn-Wall approach as well as with the values reported in literature [6]. The contribution of each of the three reaction steps is 40.3%, 13.6% and 46.1%, respectively, which correlates well with the presented mass-loss steps.
Table 2: Parameters resulting from the model-base approach using a three-step consecutive modle of nth order
| Parameter | 1st Step (Fn) | 2nd Step (Fn) | 3rd Step (Fn) |
|---|---|---|---|
| Log (PreExp) | 9.88 | 0.88 | 8.02 |
| EA (kJ/mol) | 101.3 | 30.7 | 116.6 |
| Reaction order | 1.01 | 0.91 | 0.77 |
| Contribution (%) | 40.3 | 13.6 | 46.1 |
Conclusion
The PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis mechanism of acetylsalicylic acid suggested in literature is a two-step mechanism with simultaneous evaporation of intermediates [6]. Gregory et al. found acetic acid to be the major compound released during the first mass-loss step. Furthermore, they suggest a PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis mechanism forming a variety of oligomers, as indicated by atomic mass units (amu) detected with mass spectrometry (MS) [6][7]. Along with the confirma-tion of the main gaseous products being acetic acid, salicylic acid, phenol and acetylsalicylic acid, an even more sophisticated TGA-GC-MS coupling technique was employed in order to separate and identify further PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis products [2]. All authors report a superposition of PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis and evaporation between 40% and 60% of the reaction’s progress.
In the present work, it was possible to implement these results into a model-based kinetic approach with a three-step consecutive model of nth order. The good correlation between experimental data and mathematical model is confirmed by the correlation coefficient of 0.99986. The values for the activation energy, for instance, are in good agreement with values reported in literature. Nevertheless, the model-based approach of a three-step consecutive model introduced here is surely a step beyond the iso-conversional model-free approaches based on Ozawa-Flynn-Wall or others [6], among others, since kinetic data is available independently for each individual reaction step.