Introduction
Dolomite is a mineral composed of calcium magnesium carbonate, chemically known as CaMg(CO3)2. It is an essential natural resource with significant technical importance across various industries. One prominent application is in the construction industry, where dolomite is utilized as a building material and an aggregate in concrete. Its hardness and durability make it an ideal component for road base, concrete blocks, and asphalt. Dolomite's resistance to weathering and erosion further enhances its value in infrastructure projects.
Understanding the ratio of calcium to magnesium in dolomite is crucial for optimizing its applications. Varying ratios of these elements can significantly impact the mineral's physical and chemical properties like solubility, reaction rates and crystalline structure. By knowing and controlling this ratio, manufacturers can tailor dolomite for specific applications, ensuring the desired characteristics and performance.
Results and und Discussion
To investigae the Ca/Mg ratio of dolomite, measurements were carried out with the NETZSCH STA 449 F3 Jupiter® using different gas atmospheres. A detailed compilation of the measurement conditions can be found in table 1.
Table 1: Measurement parameters
| Instrument | STA 449 F3 Jupiter® |
| Furnace | Silicon carbid furnace |
| Sensor | TG-DSC Types |
| Crucible | 85 μl Al2O3 with pierced lid |
| Temperature program | 40°C - 1200°C mit at K/min |
| Atmosphere | 70 ml/min of synthetic air or carbon dioxide |
| Sample mass | approx. 40 mg |
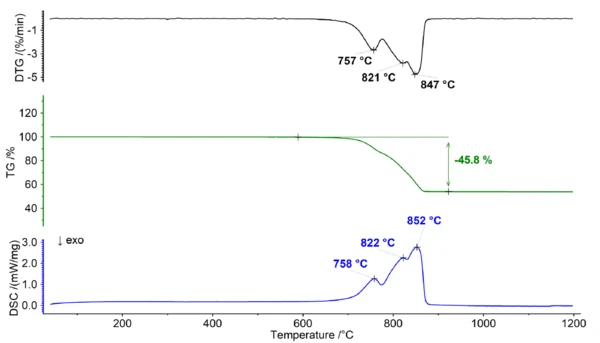
Thermal Behavior of Dolomite Under Air
When dolomite is heated in the presence of oxygen, it undergoes thermal Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition. The Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction can be represented as follows:
CaMg(CO3)2 → CaO + MgO + 2 CO2 (1)
During this Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition process (see figure 1), the solid dolomite breaks down into calcium oxide (CaO), magnesium oxide (MgO), and carbon dioxide gas (CO2). This reaction occurs at temperatures above 700°C and results in a broad mass-loss step which exhibits multiple peaks within the DTG and DSC signal. The overlapping characteristic of the mass loss significantly hinders the ability to assign distinct steps in the TGA curve, thus rendering it impossible to determine any additional information regarding the Ca/Mg ratio of dolomite.
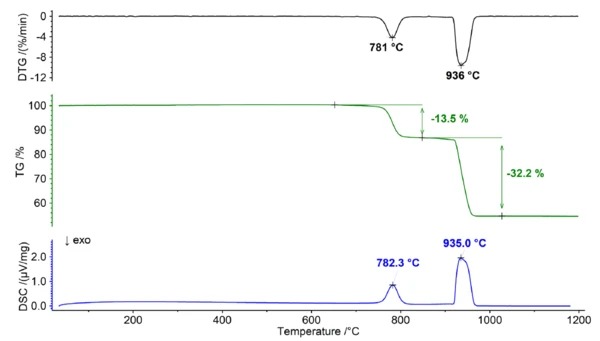
Thermal Behavior of Dolomite Under a Carbon Dioxide Atmosphere
In the presence of a carbon dioxide-containing atmosphere, dolomite exhibits different behavior due to the reversible nature of the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction and the varying stability of the carbonates involved. The Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction (1) can also be represented as two individual equilibrium reactions as follows:
MgCO3 → MgO + CO2 (2)
CaCO3 → CaO + CO2 (3)
The equilibrium between the reactants and products of the reaction is affected by various factors, including temperature and the concentrations of CO2. When carbon dioxide is consistently present, it has an impact on the equilibrium, causing a shift towards the stabilization of carbonates and resulting in a higher Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition temperature. This effect is more prominent for calcium carbonate than for magnesium carbonate due to its larger. Gibbs Energy of Formation. As a result, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of calcium carbonate and magnesium carbonate can be clearly distinguished when measured under a pure carbon dioxide atmosphere (see figure 2). This separation offers an opportunity to accurately determine the Ca/Mg ratio of the dolomite, as the initial step can be attributed to the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of MgCO3 (reaction 2), while the subsequent step corresponds to the decomposition of CaCO3 (reaction 3).
Conclusion
Selection of the optimal atmosphere significantly impacts the quality and quantity of information attainable through thermoanalytical measurements. An example of this phenomenon is given in the presented thermal analysis of a dolomite sample. In this case, the transition from a conventional air atmosphere to the slightly more unconventional choice of carbon dioxide resulted in a remarkable impact on the measured thermal properties of the analyzed dolomite. As a consequence, entirely new information like the Ca-Mg ratio became accessible.