Introduction
The presence of residual oxygen is a well known problem in thermal analysis (please refer to the terminology given in DIN 51 005). When samples are to be investigated under inert gas conditions using for example, nitrogen, argon or helium as a purge gas, the presence of residual oxygen is decisive in most cases because possible OxidationOxidation can describe different processes in the context of thermal analysis.oxidation of the sample would lead to undesired results and incorrect interpretations.
Metallic samples oxidizing on the surface exhibit an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic DSC signal as well as an increase in sample mass. The OxidationOxidation can describe different processes in the context of thermal analysis.oxidation can also be responsible for a shift in the phase transformation temperatures. Polymers or composites containing organics would partially combust in the presence of residual oxygen which would falsify the measurement result during the nominally pyrolytic Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition.
Residual oxygen in thermal analyzers is typically minimized by evacuating, backfilling and purging of the apparatus with a highly pure inert gas. This process should be repeated several times in order to minimize the oxygen concentration. The most important prerequisite for the lowest possible oxygen concentration is of course a vacuum-tight instrument. Thus, the concentration of residual oxygen depends on the vacuumtightness of the thermal analyzer, of the gas lines and of the gas connections as well as on the purity of the inert purge gas. Additional cleaning of the purge gas outside of the instrument can be helpful but does usually not yield completely satisfactory results.
The OTS® System
The OTS® system allows for an additional, efficient in-situ reduction of the oxygen concentration at the sample site. Figure 1 shows the OTS® system installed in a simultaneous thermal analyzer (STA = TGA + DSC): Below the sample and reference crucible and thus in the hot zone of the instrument is a highly temperature-resistant getter material which absorbs residual oxygen at sufficiently high temperatures. The getter material is positioned by a ceramic getter support, which is also highly temperature-resistant and does not react with the getter material. Both parts, the getter material and the ceramic getter support, are placed on the radiation shield of the TGA-DSC sample carrier.
The rotational symmetry ensures that the OTS® system is not in direct contact with the sample carrier. And due to the slitted design of the getter material and the ceramic getter support, the OTS® system can be easily mounted or removed. The inert purge gas flowing upwards fi rst comes into contact with the getter material and then with the sample. Therefore, residual oxygen which is present in the purge gas is completely absorbed by the getter material and can thus not reach the sample.
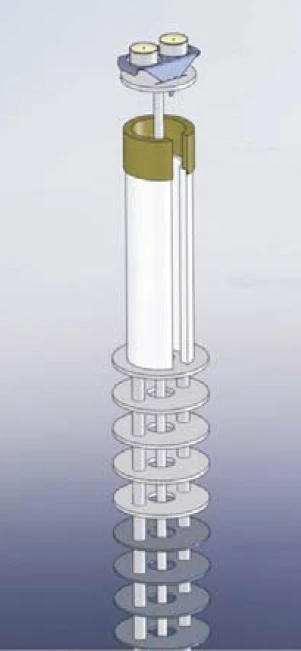
Results and Discussion
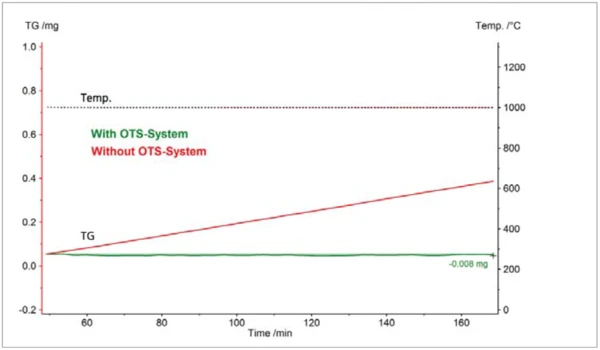
Two TGA measurements on zirconium are compared in figure 2; one with the OTS® system and the other without. Both measurements were carried out in a dynamic helium atmosphere with a nominal purity of 99.996%. The samples were held isothermally at 1000°C for about 2 hours. Without the OTS® system, the sample mass increased at a constant rate, finally arriving at 0.33 mg. This mass gain reflecting the OxidationOxidation can describe different processes in the context of thermal analysis.oxidation of the sample was avoidable with the OTS® system: The sample mass remained almost constant. From these results, it can be estimated that the OTS® system reduces the residual oxygen concentration at the sample site to below ~1 ppm.
Another example demonstrating the benefit of the OTS® system is shown in figure 3. Two nickel samples were investigated by means of an simultaneous thermal analyzer. Argon purge gas with a purity of 99.996% was used in both cases. The literature Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of nickel of 1455°C is often employed for thermometry at high temperatures. Nickel, however, is very sensitive to OxidationOxidation can describe different processes in the context of thermal analysis.oxidation which can lead to an undefined reduction of the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point and thus to incorrect thermometry. This can be seen in the measurement without the OTS® system: The sample oxidized, resulting in an increase of the TGA curve due to the mass gain. The DSC melting peak occurred already at 1443°C which is 12°C lower than the literature value. The melting enthalpy of 275 J/g is also significantly lower than the literature value of approx 300 J/g. Correct results, corresponding to literature values, were obtained with the OTS® system: The DSC melting peak was detected at 1455°C and the enthalpy of melting was 290 J/g.
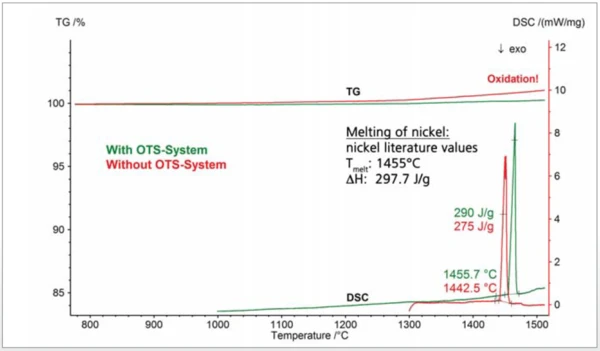
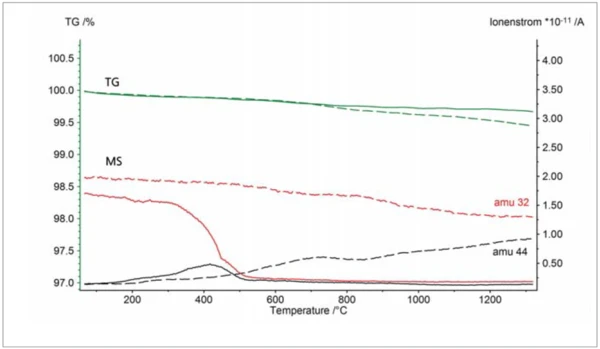
Due to the OTS® system, the sample did not oxidize significantly. This can be seen via the horizontal TGA curve, which means that the sample mass was constant during the course of the experiment. Finally, depicted in figure 4 are two TGA-MS measurements on two graphites which were again carried out in argon purge gas with a purity of 99.996%. The slight mass loss below ~600°C is most probably due to volatile hydrocarbons, whereas the mass loss at higher temperatures observed without the OTS® system reflects the partial burnup of the graphite due to residual oxygen (dashed lines): The mass spectrometer detected an increase of the signal with mass number 44 which is due to CO2 evolvement; the gradual decrease of the signal with mass number 32 reflects the corresponding consumption of residual oxygen. With the OTS® system, the sample mass remained practically constant above ~600°C which means that the sample showed no further OxidationOxidation can describe different processes in the context of thermal analysis.oxidation (solid lines). There was also no CO2 evolvement detected in that temperature range. From the oxygen signal (mass number 32), it can also be concluded that the OTS® system starts to absorb residual oxygen above ~300°C and reduces the oxygen concentration to a minimum above ~500°C.
Conclusion
The OTS® oxygen trap systems can be employed with various thermal analyzers (DSC, TGA, STA, DIL). It removes the traces of residual oxygen in the gas atmosphere inside the instrument to concentrations well below 1 ppm.