Introduction
Differential Scanning Calorimetry (DSC) does not only allow for determination of phase transformation temperatures but also for quantification of transformation enthalpies. The samples are generally analyzed in aluminum crucibles with a pierced lid under an atmospheric pressure in a constant purge gas flow. With modified instruments – where the measuring cell is installed in an autoclave (a so-called pressure DSC) – measurements in a pressure range between 5 kPa and 15 MPa are additionally possible [1]. In this application note, liquids are analyzed with regard to their evaporation behavior in this pressure range.
Since the evaporation of liquids before reaching the boiling temperature and the equilibrium between liquid and gas during boiling are critical parameters, which can have negative influence on the reaction and the later evaluation, special crucibles were employed for these measurements. Good experience was made with this cold-welded aluminum crucibles with a small defi ned hole with a diameter of 50 μm.
The Antoine equation describes the relationship of the saturation vapor pressure of a pure substance and temperature:
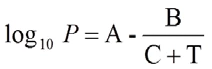
where P is the pressure in bar, T is the temperature in K and variables A, B and C are component-specific constants. These, however, cannot be used to describe the entire process of the boiling point curve of a substance. Therefore, there are several parameter sets for the range from the triple to the critical point.
The following table summarizes the investigated liquids water, cyclohexane, ethyl acetate and isopropanol for the validity range of the literature data used:
Table 1: Temperature range and coefficients of the Antoine equation [3, 4, 5]
| Substance | Temperature Range [K] | Temperature Range [°C] | A | B | C |
|---|---|---|---|---|---|
| H2O | 313 ... 385 | 40 ... 112 | 6.1680 | 1397.2 | -48.097 |
| C6H12 | 323 ... 523 | 50 ... 250 | 4.1398 | 1316.5 | -35.581 |
| C4H8O2 | 288 ... 348 | 15 ... 75 | 4.2280 | 1245.7 | -55.189 |
| C3H8O | 395 ... 508 | 122 ... 235 | 4.5779 | 1221.4 | -87.474 |
Results
Water
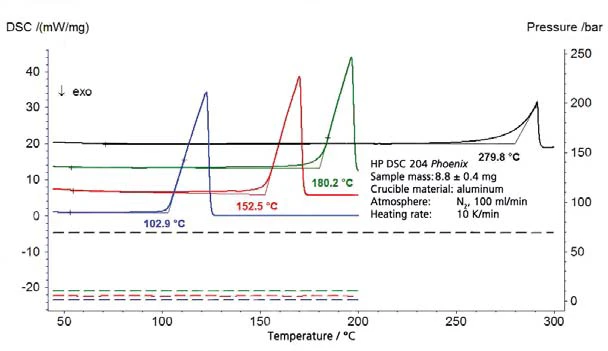
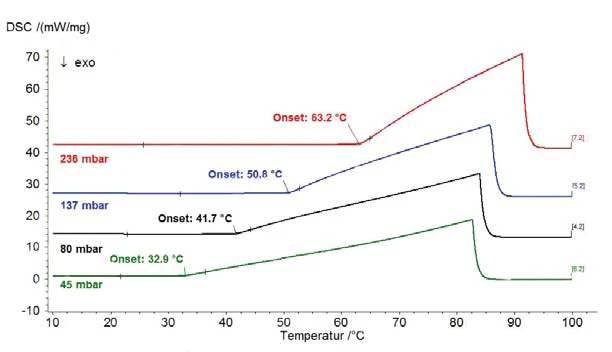
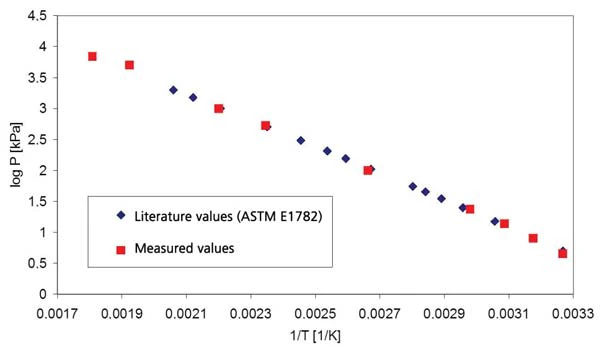
The results on destilled water for the pressure range above atmospheric pressure are shown in figure 1 (pressure shown with dashed lines); figure 2 depicts the pressure range between 45 mbar and 236 mbar. Figure 3 shows the good agreement of the literature data from [2] (ASTM E782) with all determined measurement values.
Cyclohexane
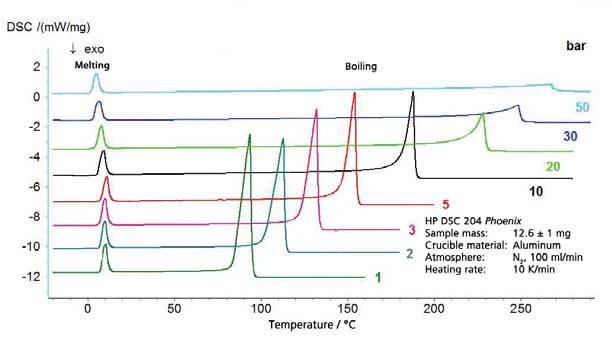
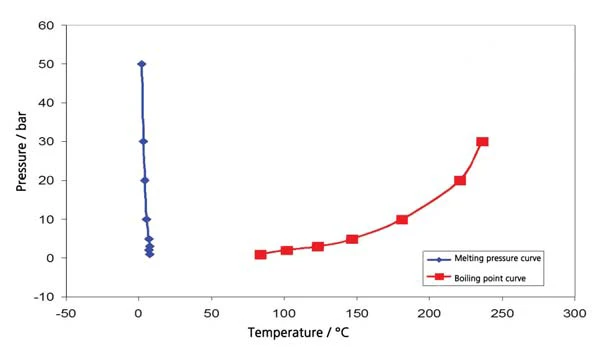
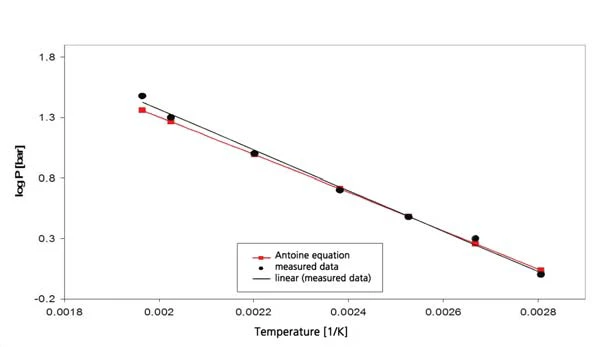
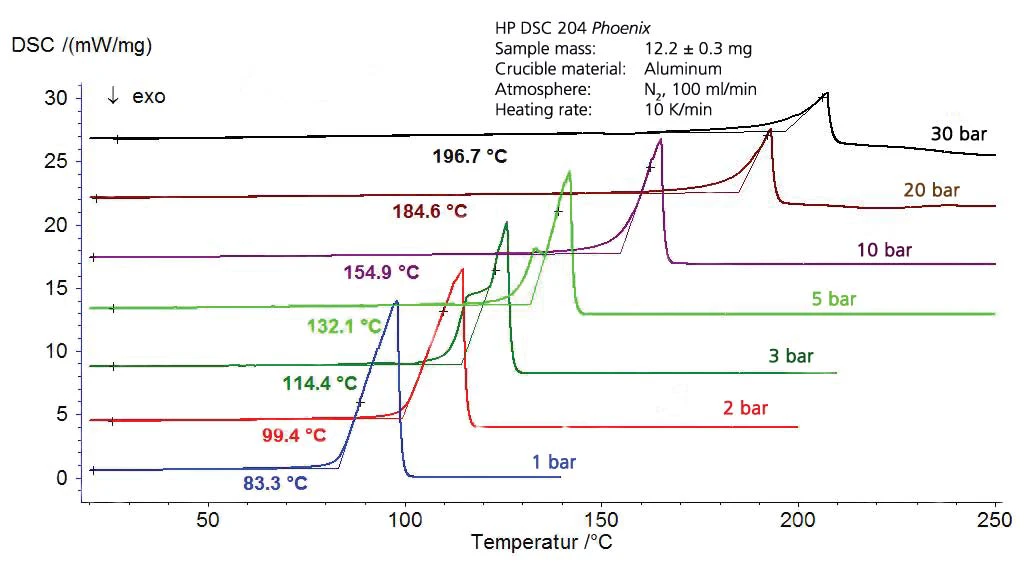
The results for cyclohexane in the temperature range between -20°C and 300°C (figure 4) include both boiling and melting. This results in the segment of the phase diagram, presented in figure 5. Figure 6 depicts the comparison with literature [3].
Ethyl Acetate
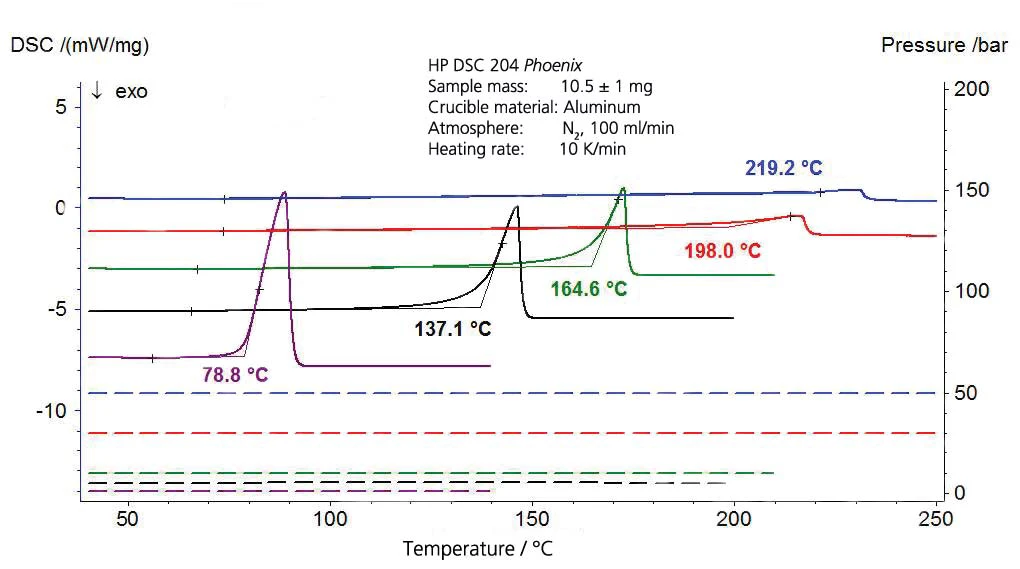
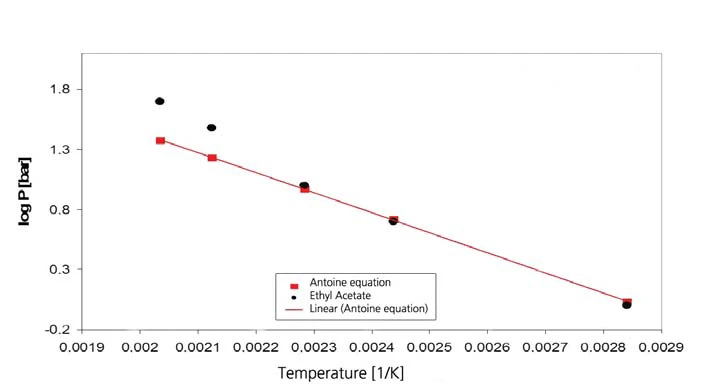
The results for ethyl acetate are shown in figures 7 and 8. The literature values, however, are extrapolated values since the validity range of the Antoine equation from [4] is limited to the interval between 15°C and 75°C (288 K to 348 K, corresponding to the reciprocal temperature values of 0.00347 to 0.00287).
Isopropanol
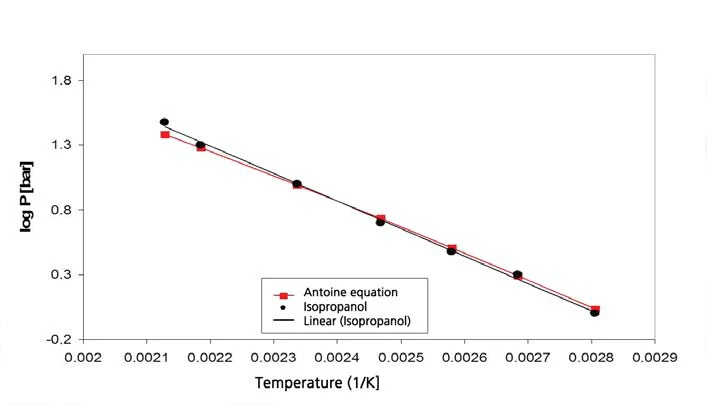
Figures 9 and 10 show the measurement results and the comparison of literature values [5] for isopropanol.
Summary
Differential Scanning Calorimetry (DSC) – combined with the possibility of pressure variations within the measuring cell – allows for the investigation of the pressure-dependence of phase transformations. The results for the liquid-gaseous transition of the investigated substances, water, cyclohexane, ethyl acetate and isopropanol are in very good agreement with literature.