Introduction
Moisture may affect the properties of a wide range of active ingredients and excipients in terms of their stability, Crystallinity / Degree of CrystallinityCrystallinity refers to the degree of structural order of a solid. In a crystal, the arrangement of atoms or molecules is consistent and repetitive. Many materials such as glass ceramics and some polymers can be prepared in such a way as to produce a mixture of crystalline and amorphous regions.crystallinity, bio-availability, etc. One method for determining the influence of humidity on a substance’s behavior is dynamic vapor Sorption ProcessSorption is a physical and chemical process by which a substance (typically a gas or liquid) accumulates within another phase or on the phase boundary of two phases. Depending on the place of accumulation, a differentiation is made between absorption (accumulation in a phase) and adsorption (accumulation at the phase boundary).sorption (DVS), in which the mass changes in the sample are measured for different solvent vapor amounts, e.g., water vapor. [1]
Such measurements can be carried out with an STA (simultaneous thermal analyzer) connected to a modular humidity generator (figure 1). In the following, a dynamic water Sorption ProcessSorption is a physical and chemical process by which a substance (typically a gas or liquid) accumulates within another phase or on the phase boundary of two phases. Depending on the place of accumulation, a differentiation is made between absorption (accumulation in a phase) and adsorption (accumulation at the phase boundary).sorption measurement was carried out on micro-crystalline cellulose (MCC, chemical structure in figure 2). This substance is used in tablet formulations as filler and binder. [2]


Measurement Conditions
The experimenal conditions are summarized in table 1.
Table 1: Conditions of the test
| Device | STA 449 F3 Nevio connected with the humidity generator |
|---|---|
| Sample | Micro-crystalline cellulose |
| Sample mass | 41.22 mg |
| Sample holder | Plate made of alumina, Ø 17 mm |
| Temperature program | IsothermalTests at controlled and constant temperature are called isothermal.Isothermal 44°C, nitrogen atmosphere, relative humidity (RH) increased from 0 to 80% |
Measurement Results
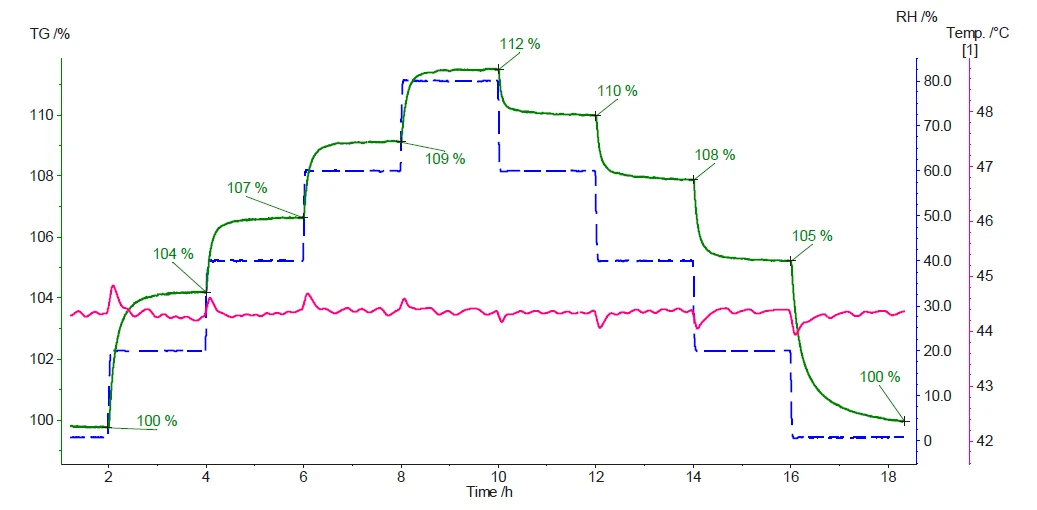
Figure 3 displays the measured sample mass and temperature during the experiment.
The results demonstrate the strong hygroscopic nature of the micro-crystalline cellulose. The first increase in relative humidity from 0% to 20% (blue dashed curve) induces a mass increase of 4% (green curve). The subsequent steps show that the higher the relative humidity, the higher the mass gain. As soon as the humidity level decreases, the absorbed and/or adsorbed water is released, resulting in a mass loss. When a completely dry atmosphere is finally reached at the end of the measurement, the amount of water absorbed and/or adsorbed will have been quantitatively released. This can be confirmed by having arrived back at the initial sample mass (100%).
Each change in relative humidity level is associated with a peak in the sample temperature curve (pink curve). This is due to the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal and endothermal natures of the Sorption ProcessSorption is a physical and chemical process by which a substance (typically a gas or liquid) accumulates within another phase or on the phase boundary of two phases. Depending on the place of accumulation, a differentiation is made between absorption (accumulation in a phase) and adsorption (accumulation at the phase boundary).sorption and desorption of water, respectively.
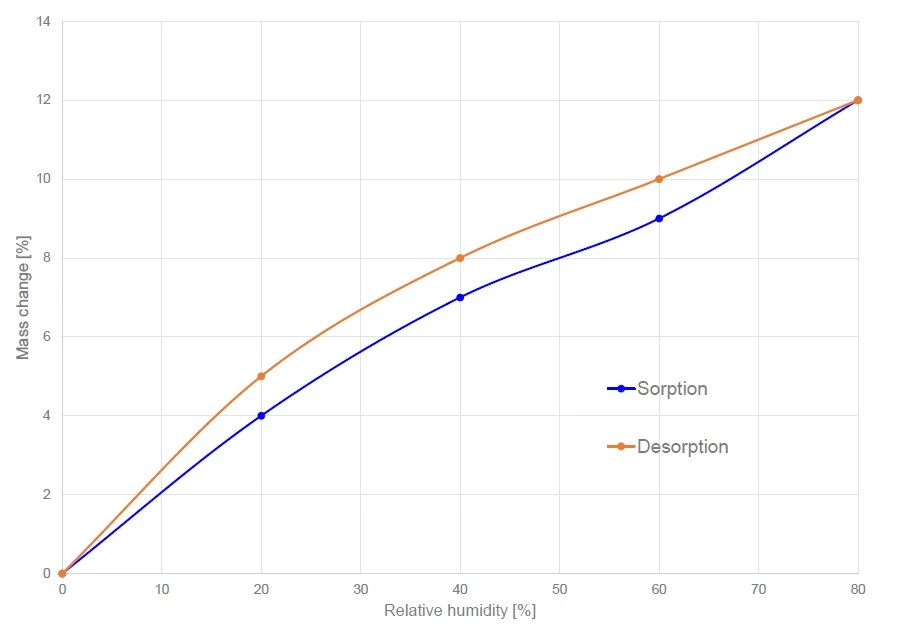
The mass gain and loss after reaching equilibrium are given in figure 4 for all measured relative humidity levels between 0% and 80%. The maximum mass increase amounts to 12% for a relative humidity of 80%. Microcrystalline cellulose exhibits Sorption ProcessSorption is a physical and chemical process by which a substance (typically a gas or liquid) accumulates within another phase or on the phase boundary of two phases. Depending on the place of accumulation, a differentiation is made between absorption (accumulation in a phase) and adsorption (accumulation at the phase boundary).sorption hysteresis, i.e., the water amount in the sample is higher during desorption than during Sorption ProcessSorption is a physical and chemical process by which a substance (typically a gas or liquid) accumulates within another phase or on the phase boundary of two phases. Depending on the place of accumulation, a differentiation is made between absorption (accumulation in a phase) and adsorption (accumulation at the phase boundary).sorption (see figure 4) but, ultimately, the starting point and ending point of the sorption/desorption cycle are identical.
This hysteresis phenomenon is typical for many porous materials. Chen et al [3] showed that the water-cellulose bonds formed during swelling of cellulose do not break upon desorption at the same chemical potential.
Conclusion
STA connected to a humidity generator allows for measurements of dynamic water sorption and desorption. The measurements on micro-crystalline cellulose highlight the hysteresis of the process: The moisture content is higher during desorption than during sorption. This phenomenon is typical for many porous material.