Introduction
Many pharmaceutical solids exhibit PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism, i.e., the ability of a substance to exist in different crystalline forms; therefore, they have different molecular arrangements in the crystal lattice. Due to their structural differences, polymorphs may have different solid properties, such as DensityThe mass density is defined as the ratio between mass and volume. density, color, Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point, solubility, mechanical properties, etc. Consequently, the polymorphic form can have significant influence on pharmaceutcial processing and can, for example, affect grinding, granulation and tabletability [1].
Paracetamol, which is used for the treatment of pain and fever, is also a polymorph, i.e., it exists in different crystalline forms. Each of these modifications behaves differently, particularly with regard to thermodynamic stability and compression ability. This last property influences the tabletability of paracetamol, i.e., its capacity to be transformed into a tablet under the effect of pressure. It has been proven that orthorhombic paracetamol (also called form II) can be used for direct compression. Its better tabletability than that of the monoclinic form of paracetamol (form I) is due to the presence of parallel sliding planes in the orthorhombic crystalline structure. [5, 6]
However, the monoclinic form is the most frequently used commercially [4], probably because of its better thermodynamical stability. Since the modification of paracetamol is closely associated with its properties, it is essential to know in which form it is present before processing, particularly for the production of tablets that require compaction. In the following, the modification of paracetamol is determined by means of differential scanning calorimetry.
Measurement Conditions
1.69 mg of paracetamol was measured in a sealed aluminum crucible with a pierced lid. This sample was heated twice to 200°C at a heating rate of 10 K/min. Between the two heating runs, it was cooled down at a controlled rate of 10 K/min.
Test Results
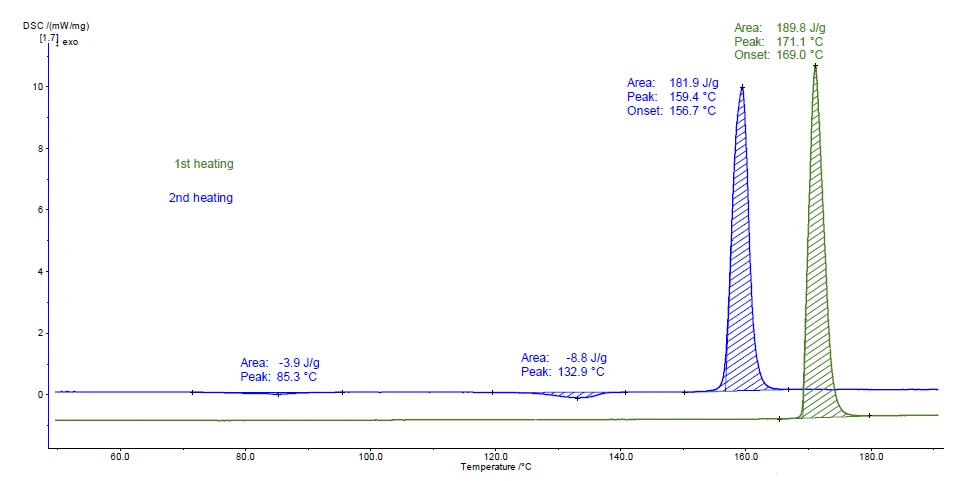
Figure 2 displays the measurement curves for the two heating runs. The peak detected at 169°C (onset temperature) during the first heating (green curve) is in very good accordance with the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point value of the crystalline modification I (see table 1).
Tab 1: Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting temperature of the different modifications of paracetamol [2, 3]
Modification | I | II | III |
|---|---|---|---|
| Metlting temperature [°C] | 169 | 156 | < 156 |
The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peaks at 85°C and 133°C (peak temperature) occurring in the second heating indicate CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization. The modification formed melts afterwards at 156.7°C (onset temperature); this is typical for the crystalline form II.
Conclusion
Solid-state characterization is of central importance for the pharmaceutical industry since drugs are predominantly produced as solids. Selection of the optimal solid form is a critical aspect in the development of drugs as they can exist in more than one form of crystal structure. This polymophism shows different physical properties which influence not only biopharmaceutical properties but also tabletability.
A single DSC heating run to the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature allows for identification of the modification of paracetamol being investigated, allowing for estimation of its behavior during compression.