Introduction
Blends consisting of PET and PC exhibit significantly better mechanical properties and processibility than each homopolymer individually. Knowledge of the ratio of each polymer in a PET/PC blend is crucial because it influences the properties of the product. In this work, modulated differential scanning calorimetry is used to evaluate the amount of each polymer in three PET/PC blends.
DSC Measurements (Conventional)
Experimental
The samples tested consisted of three blends of polycarbonate (PC) and polyethylene terephthalate (PET) in different ratios. They were free of additives or any other component. They were produced in exactly the same way and stored under the same conditions prior to the measurements. In the following, the three samples are designated PET/ PC1, PET/PC2 and PET/PC3. The measurement conditions are summarized in table 1.
Table 1: Experimental conditions of the conventional DSC measurements
| Device | DSC 204 F1 Phoenix® (NETZSCH-Gerätebau GmbH) | |
|---|---|---|
| Atmosphere | Nitrogen (flow rate: 40 ml/min) | |
| Sample masses | Between 11 and 12 mg | |
| Crucible | Cold-welded aluminum crucibles with pierced lids | |
| Temperature program | ↗ 0°C ... 280°C at a heating rate of 10 K/min ↓ 280°C ... 0°C at a heating rate of 20 K/min ↗ 0°C ... 280°C at a heating rate of 10 K/min | |
Results and Discussion
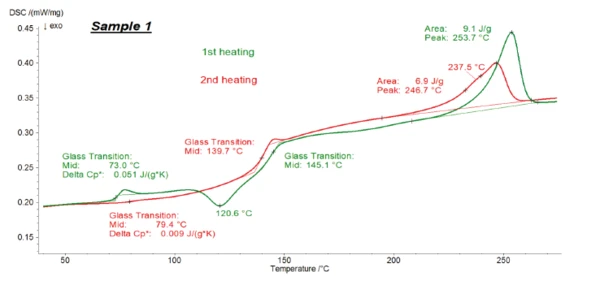
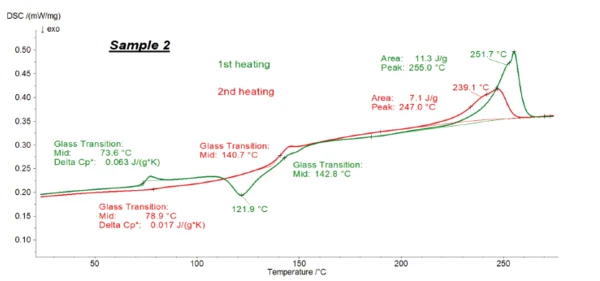
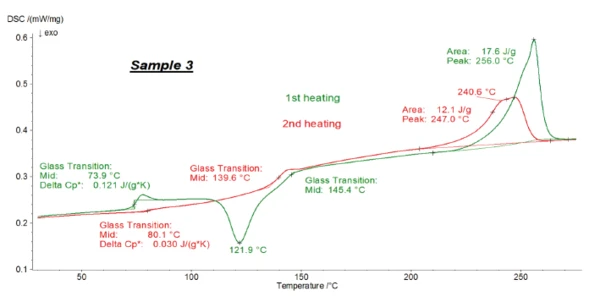
Figures 1, 2 and 3 present the transformation energetics of PET/PC1, PET/PC2 and PET/PC3 during the two heating runs. The first heating run is depicted in green; the second in red.
The DSC curve for the first heating shows the history of the polymer before the measurement: It reflects the preparation, cooling and storage conditions, etc. In contrast with that, the second heating helps identify the polymer. Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting of the polymer in the first heating “erases” its history. After a controlled cooling under defined conditions, the second heating provides information about the identity of the sample.
In both heating cycles for all samples, the typical EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic step (Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition) of PET was detected between 70°C and 85°C, along with its melting peak between 200°C and 270°C. For all samples, the ΔSpecific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.cp step of PET was smaller in the second heating than in the first heating, which indicates the formation of a lesser amount of the amorphous phase during cooling. The post-CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization peak of PET at 120.6°C (peak temperature), only detected in the first heating, confirms this: This effect is due to the reorganization of the amorphous structure to build crystallites and is only detected for low-crystalline PET. The Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of polycarbonate is detected at approx. 140-145°C. In the first heating, it overlaps the post-CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization peak of PET.
Therefore, accurate evaluation of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of polycarbonate is not possible by means of conventional DSC.
As explained above, the second heating is typically used to identify a polymer substance. However, the polymer ratios are calculated by evaluating the ΔSpecific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.cp inherent to the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition. The ΔSpecific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.cp steps of PET in all three cases are higher in the first than in the second heating, so it is more accurate to evaluate them using the first heating. Furthermore, the delivered samples had the same thermal history and were prepared in exactly the same way for the DSC measurements. For these reasons, the first heating was used for evaluating the amount of each polymer in the blends.
Temperature-Modulated DSC Measurements
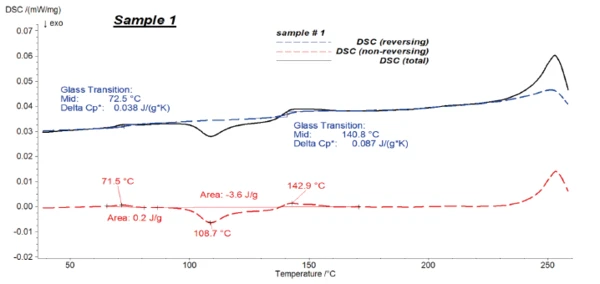
In a modulated DSC measurement, the temperature signal is no longer linear, but sinusoidal: An oscillating heating rate of defined amplitude and period is applied onto the underlying heating rate. As a result, the DSC signal is separated into two parts: the oscillating part, which is the so-called reversing signal giving information about the processes that oscillate with the temperature, e.g., heat capacity changes; and the non-reversing heat flow, which is related to time-dependent processes, e.g., evaporation or CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization (see also table 2).
Here, temperature-modulated tests are carried out to separate the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization peak of PET from the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of PC. This allows for accurate evaluation of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transitions.
Experimenal
Table 3 summarizes the conditions for the modulated tests.
Results and Discussion
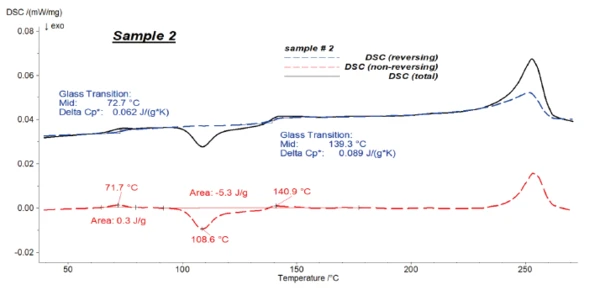
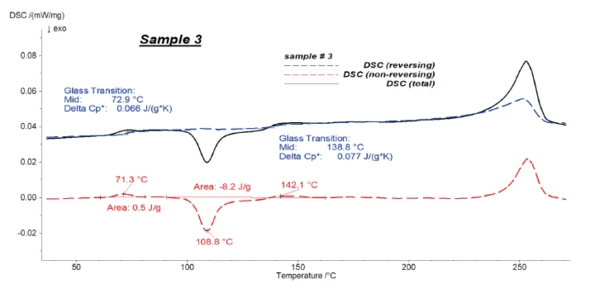
Figures 4 to 6 present the results of the modulated measurements for the three PET/PC blends. As expected, the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of both polymers is visible in the reversing signal, whereas post-crystallisation of PET can be seen in the non-reversing signal. Furthermore, the EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effects after each Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition, which are due to the RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation effects of the samples, are also only visible in the non-reversing signal.
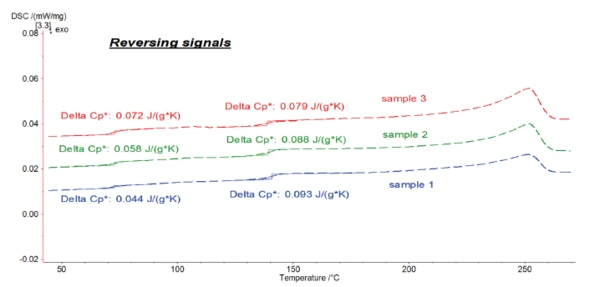
It was possible to evaluate ΔSpecific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.cp during the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of the samples with high accuracy in the reversing parts.
Figure 7 shows the reversing signals for all of the samples.
Table 2: Typical distribution of the measured effects into reversing and non-reversing signals
| Reversing signal | Non-reversing signal (time-dependent process) | |
|---|---|---|
| Glass transition | ||
| Solid/solid transition | CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization, post-crystallizaiton | |
Evaporation | ||
Curing | ||
Table 3: Experimental conditions for the modulated DSC measurements
| Device | DSC 204 F1 Phoenix® (NETZSCH-Gerätebau GmbH) | |
|---|---|---|
| Atmosphere | Nitrogen (flow rate: 40 ml/min) | |
| Sample masses | Between 11 and 12 mg | |
| Crucible | Aluminum crucibles with pierced lid | |
| Temperature program | 20°C - 280°C Heating rate: 1.5 K/min Amplitude: 0.5 K Period: 120 s | |
With this accurate evaluation of ΔSpecific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.cp for the three blends, the amount of PET and PC in each sample can be determined using the followig equations:
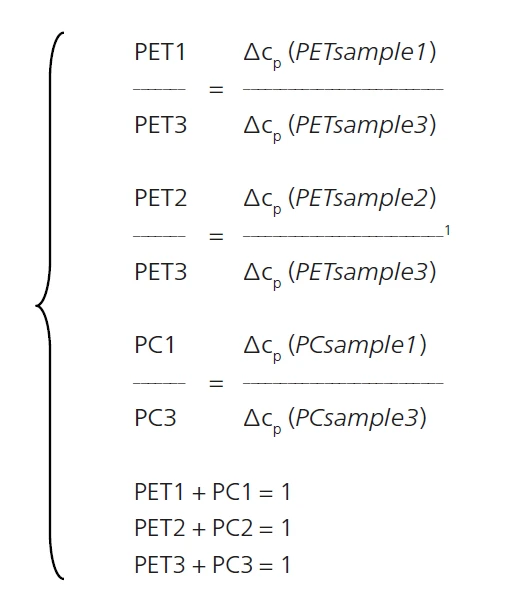
In which PET1, PET2, PET3 are the PET content levels in samples 1, 2 and 3; and PC1, PC2 and PC3 are the PC content levels in samples 1, 2 and 3. These calculations can, of course, only be carried out accurately if the samples include no other component (filler, color batch, etc.) and the thermal history is identical for the three blends.
The following calculations can then be determined:
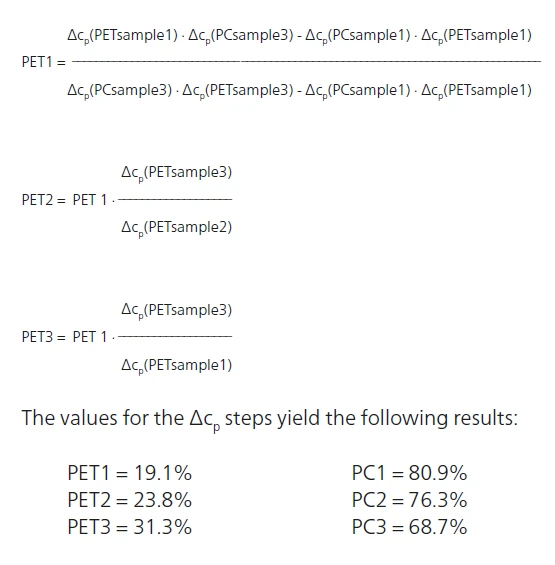
Conclusion
Three PET/PC blends were measured using the DSC 204 F1 Phoenix®. In standard DSC measurements (without modulation), the Δcp step of polycarbonate is overlapped with the post-CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization peak of PET; therefore, accurate evaluation is not possible.
Additional measurements were carried out by employing temperature-modulated DSC in order to separate the two effects. The Δcp steps allow for accurate determination of the content levels of PET and PC in each sample.