Introduction
Paints, adhesives, printing inks, and potting compounds are increasingly cured at moderate temperatures (often at room temperature) by means of ultraviolet (UV) radiation. Along with the energy-saving aspect – in comparison with thermal curing – the high processing speeds of UV-induced cross-linking and the eco-friendliness of UVreactive systems are of main interest for industrial applications. Since the energy input is brief, objects coated in this manner barely undergo heating. That is why this technique can even be employed for the surface treatment of heatsensitive substrates such as plastic films, wood, and paper. Also, UV-cured paint films generally exhibit high scratch and chemical resistance.
In order to realize the afore-mentioned advantages of this method and generate high-quality products, if optimization of the UV curing formulations is necessary, and the optimal irradiation times and radiation intensities must be determined. Photo-calorimeters, sometimes also designated Photo-DSC or UV-DSC, are ideal for the investigation of light-active substances and their curing behavior.
UV Curing is Very Fast
UV curing is generally complete within seconds. Reaction mechanisms typically involve cationic or radical polymerizations, i.e., cross-linking triggered by an initiator that decomposes under the influence of ultraviolet light, causing either an Ionic or radical chain reaction.
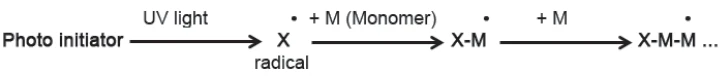
The basic principles of both reaction types are similar [1]. Most UV coatings employ radical polymerization (see schematic in figure 1). Radicals formed during Reakcja rozkładu (dekompozycji)A decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the photoinitiator react, for example, with the double bonds of monomers, generating new radicals that sustain the polymerization. As curing progresses, the material becomes more viscous, limiting the ability of the radicals and double bonds to diffuse together, so that the reaction rate decreases.
One advantage of cationic polymerization over radical polymerization is that cationic polymerizations are less sensitive to the influence of oxygen.
Setup and Operating Mode of the UV-DSC Based on the DSC 204 F1 Phoenix®
Differential Scanning Calorimetry (abbreviated DSC) is a thermoanalytical method in which the heat flow difference between a sample and a reference, subjected to a controlled temperature program, is quantitatively determined (definition based on DIN 51 007, ISO 11357 - 1 or ASTM E 472).
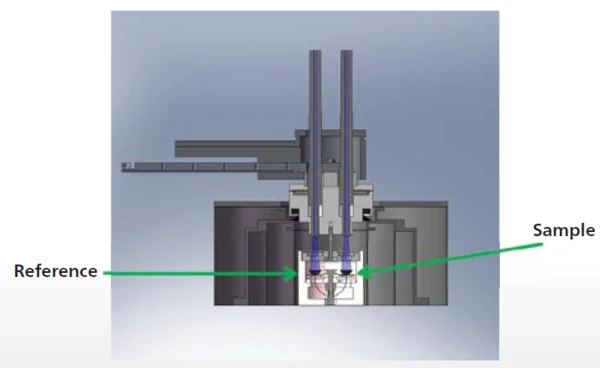
Figure 2 shows the NETZSCH DSC 204 F1 Phoenix® heat-flux calorimeter (see also schematic setup with UV attachment [2], figure 3). Both the sample and reference are located in one furnace and are irradiated simultaneously (depicted in blue). The fiber optics is firmly installed in the lid so that reproducible distances between the fiber optics and the sample and reference are guaranteed. The DSC measurement software communicates with the UV lamp, triggering its pulses and controlling the pulse length and intensity automatically.
During the course of a measurement, the signals detected are the sample temperature and heat flow difference. By integrating the heat flow signal, the heat of curing can be determined, providing meaningful data for development or process optimization.

Optimization of Exposure Time and Degree of Cure by Means of UV-DSC
During the development process of adhesives, inks, etc, it is important to find the optimal exposure time, i.e., the exposure time necessary to reach the desired degree of cure, and hence, the desired material properties. Degree of cure is of primary interest for in-process testing as well as for quality control.
In a standard UV-DSC measurement, the sample is initially heated to the desired reaction temperature (this is 30°C in figure 4) and, after a short temperature equilibration phase, irradiation is started. Multiple IsothermalTests at controlled and constant temperature are called isothermal.isothermal segments, each including a single lamp pulse, are generally programmed since multiple pulses of a defined length and intensity allow the sample curing to be monitored to completion. The UV lamp is usually triggered a few seconds after the start of each segment.
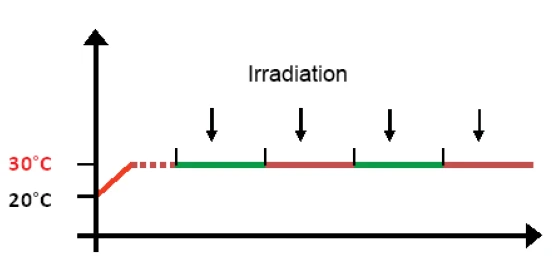
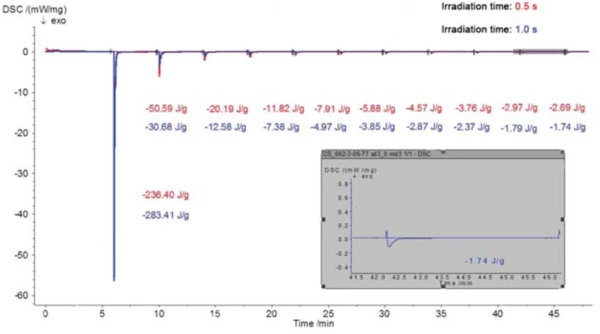
Figure 5 shows the results of two investigations (presented in red and blue) on a commercially available acrylate-based coating with different irradiation times (0.5 s and 1 s). As expected, in both cases, the majority of the EgzotermicznyA sample transition or a reaction is exothermic if heat is generated.exothermal reaction occurs during the first irradiation phase; the reaction enthalpies are slightly different for the different irradiation times, however with the longer 1 s pulse leading to a slightly higher enthalpy of -283.4 J/g compared to -236.4 J/g for the 0.5 s pulse. This difference is almost made up in the following irradiation segments. This means that, at a constant irradiation intensity, a higher irradiation time (blue curve) in the first segment results in a higher partial degree of cure and smaller post-curing in the following segments. An even clearer graphical representation of the data is shown in figure 6.
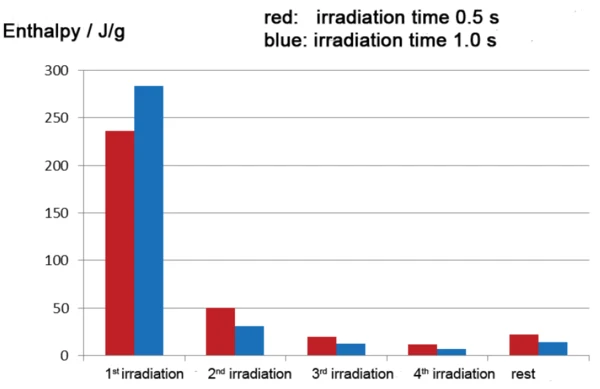
Starting at approximately the 10th irradiation phase, the peak areas in the DSC measurement associated with each pulse barely change. The constant residual peak area once the curing is complete is due to diff erential heating of the samples versus the reference by the radiation. Calculation of the total enthalpy of the curing process requires that this residual enthalpy to be subtracted from the enthalpy contribution of each peak included in the calculation.
If the enthalpy of the first irradiation phase is related to the total enthalpy, a degree of cure of approximately 82% is calculated for the first 1 s pulse and degree of cure of approximately 67% is calculated for the first 0.5 s pulse. Depending on the targeted degree of cure for practical use, a single irradiation step of 1 second exposure length could possibly be sufficient – assuming that the thickness of the process sample is comparable to the thickness of the DSC sample.
Oxygen as an Inhibitor for Acrylate Systems
For the reaction process of many photo-cured paint systems, oxygen gas plays a decisive role. For acrylate systems, oxygen acts as an inhibitor. Its mechanism of action was already described by G.V. Schulz and G. Henrici [3] in the 1950’s. In the presence of oxygen, peroxy radicals form, leading to oxygen incorporation into the polymer. This results in relatively short copolymer chains [4].
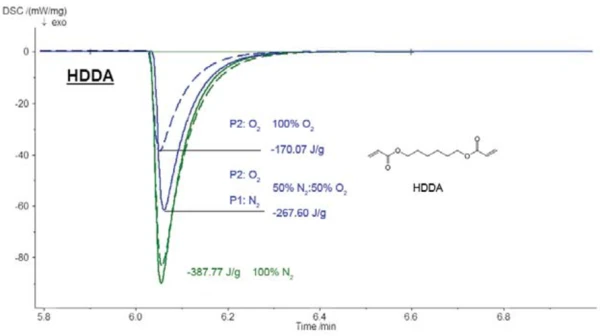
Figure 7 shows the influence of oxygen on the photocuring of hexandiol diacrylate (HDDA). The reaction enthalpy decreases signifi cantly with increasing oxygen concentration.
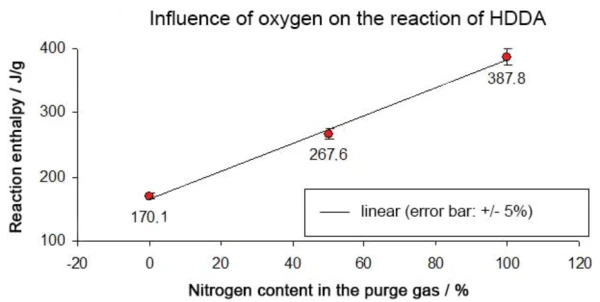
The reaction enthalpy in a pure nitrogen atmosphere was -388 J/g, as compared to -268 J/g in a mixture of 50% nitrogen and 50% oxygen and -170 J/g in a pure oxygen atmosphere. This results in a linear correlation between the reaction enthalpy and oxygen content (see figure 8).
Conclusion
The NETZSCH DSC 204 F1 Phoenix® with UV lamp accessories features easy handling. The gastight design allows for precise control of the atmospheric composition in the sample chamber; this is of paramount importance with regard to the content of residual oxygen in the purge gas. The UV lamp is controllable with the DSC measurement software. Parameters such as irradiation time and intensity can thus be pre-selected in the DSC measurement program. For a high number of measurements, the automatic sample changer (ASC) can also be used in connection with the UV attachment.
Differential Scanning Calorimetry (DSC) in combination with sample irradiation by a UV lamp is ideally suited for the simple and rapid characterization of photo-initiated curing processes. The results of such measurements offer insights into curing mechanisms, and yield important information for the improvement of formulations (inhibitors, photo-initiators, fillers) and for process control.
This article was published in June 2013 edition of Laborpraxis (with a reduced amount of figures).