Introduction
An important attribute of polymers, or hydrocarbons in general, is their aging behavior. The impact of environmental influences such as oxygen, UV radiation, temperature and humidity can affect the quality of both raw materials and products during application or storage. Hence, there is a need for information about storage stability or aging behavior with respect to incoming goods inspection, quality assurance and the shelf life of organic substances. Irrespective of which chemical reaction mechanisms are behind the aging processes, they all ultimately lead to degradation of the material. This scission of molecules or molecular chains results in increasingly smaller fragments; the further the aging progresses, the smaller the molecules become. The shorter molecular chains, in turn, exhibit higher reactivity to oxygen, so their resistance to oxygen is reduced.
All hydrocarbons react with oxygen in a heavily ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal OxidationOxidation can describe different processes in the context of thermal analysis.oxidation reaction while carbon dioxide (CO2) and water (H2O) are formed. These OxidationOxidation can describe different processes in the context of thermal analysis.oxidation reactions – along with the melting and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization behavior [1] – can be very easily observed by means of Differential Scanning Calorimetry (DSC). Based on the reaction behavior, the current state of a substance with regard to aging can be determined. A series of samples is generally investigated under identical conditions and the results are compared. Such a measurement series is particularly meaningful when samples of differing ages are compared with a non-aged sample. This is the reason why so many measuring specifications exist for determining the aging behavior (OxidationOxidation can describe different processes in the context of thermal analysis.oxidation behavior) of fats, oils, waxes, or polymers and hydrocarbons in general with the help of DSC [2].
Measurement Specifications
The OxidationOxidation can describe different processes in the context of thermal analysis.oxidation reaction of hydrocarbons with oxygen is either – as in the case of oils – a liquid-gas reaction, or – as in the case of polymers – a solid-gas reaction. In either case, the reaction surface – i.e., the sample surface – is particularly important. The sample mass and sample preparation methods are therefore also defined in the measurement specifications, as are the crucible material or crucible geometry, the reaction gas (synthetic air or pure oxygen), the purge gas rate, the heating rate and the IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature.
Depending on the sample material and reactivity, the relevant standards recommend experiments at either a constant temperature (Oxidative-Induction Time (OIT) and Oxidative-Onset Temperature (OOT)Oxidative Induction Time (isothermal OIT) is a relative measure of the resistance of a (stabilized) material to oxidative decomposition. Oxidative-Induction Temperature (dynamic OIT) or Oxidative-Onset Temperature (OOT) is a relative measure of the resistance of a (stabilized) material to oxidative decomposition.OIT = Oxidative-Induction Time) or a constant heating rate (Oxidative-Induction Time (OIT) and Oxidative-Onset Temperature (OOT)Oxidative Induction Time (isothermal OIT) is a relative measure of the resistance of a (stabilized) material to oxidative decomposition. Oxidative-Induction Temperature (dynamic OIT) or Oxidative-Onset Temperature (OOT) is a relative measure of the resistance of a (stabilized) material to oxidative decomposition.OOT = OxidationOxidation can describe different processes in the context of thermal analysis.Oxidation Onset Temperature). There are also measurement specifications for where a constant gas flow rate is employed under atmospheric pressure or at an increased oxygen pressure of 35 bar (3.5 MPa). Since the purge gas for these tests – oxygen – is also a reaction gas, the oxygen pressure employed is not only a physical measurement parameter, but also a measure for the concentration of one of the reactants. The reaction rate increases with rising oxygen pressure; measurements at an increased oxygen pressure are therefore accelerated aging tests. The increased pressure is also advantageous because it suppresses disturbing caloric influences – for example, ones which might have arisen due to evaporation of the liquids being investigated. In the following table, the most common standards are shown in relation to the various measurement conditions:
Table 1: Measurement conditions for the most common standards with regard to temperature and pressure control
1 bar | 35 bar | |
|---|---|---|
| IsothermalTests at controlled and constant temperature are called isothermal.Isothermal | ASTM D3895-07 ISO 11357-6 | ASTM D6186-08 ASTM D5483-05 ASTM D5885-05 ASTM E1858-08 |
| Dynamic | ASTM E2009-08 ISO 11357-6 | ASTM E2009-08 |
This application note was written using primarily the measurement conditions suggested in ASTM E2009-08, since that standard specifically relates to cooking oils and recommends dynamic measurements both under atmospheric pressure and increased pressure. The choice of crucible, however, was made based upon the recommendations in ASTM D6186-08 and ASTM D5483-05, which favor “SFI” crucibles (SFI = Solid Fat Index) made of aluminum over simple cylindrically shaped aluminum crucibles for the investigation of lubricants. This special crucible shape ensures that a liquid sample’s contact area to the crucible bottom remains unchanged during the measurement, since it prevents the liquid substances from creeping up onto the lateral surfaces due to capillary force. Figure 1 shows the deepened edge zones of the crucible bottom which are characteristic of SFI crucibles.
The production of these crucibles using cylindrically shaped aluminum crucibles (order no. NGB810405) with the help of a special pressing tool (order no. 6.240.10-84.0.00) – as well as the use of SFI crucibles in Oxidative-Induction Time (OIT) and Oxidative-Onset Temperature (OOT)Oxidative Induction Time (isothermal OIT) is a relative measure of the resistance of a (stabilized) material to oxidative decomposition. Oxidative-Induction Temperature (dynamic OIT) or Oxidative-Onset Temperature (OOT) is a relative measure of the resistance of a (stabilized) material to oxidative decomposition.OIT investigations – are all described in literature [3].
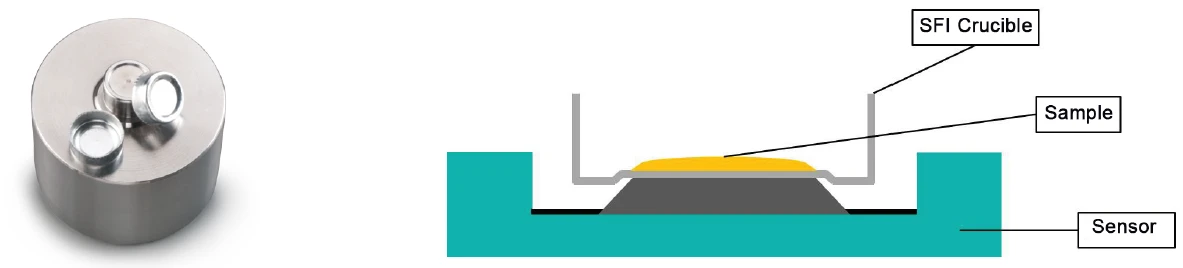
Experimental
The OxidationOxidation can describe different processes in the context of thermal analysis.oxidation behavior of the cooking oils derived from sunflower seed, walnut, rapeseed (canola), peanut, pumpkin seed, pistachio seed and olive was investigated using a NETZSCH DSC 204 HP with t-Sensor. Oxygen was used as purge and pressure gas; the purge gas rate amounted to 100 ml/min. The oils were pipetted into open aluminum crucibles (SFI) in such a way as to ensure that the central bottom areas of the crucibles were completely wetted. The measurement parameters and sample masses are summarized in table 2.
Table 2: Measurement conditions
Dynamic | IsothermalTests at controlled and constant temperature are called isothermal.Isothermal | |
|---|---|---|
| Measuring instrument | HP-DSC 204 | HP-DSC 204 |
| Sensor | t-sensor | t-sensor |
| Cooling | GN2, auto | GN2, auto |
| Crucible | Al open, SFI | Al open, SFI |
| Atmosphere | Oxygen (99.6%) | Oxygen (99.5%) |
| Gas flow rate | 100 ml/min | 100 ml/min |
| Pressure | 35 bar (3.5 MPa) | 35 bar (3.5 MPa) |
| Heating rate | 10 K/min | 100 K/min |
| Sample masses | 3.05 mg (±0.03) | 3.05 mg (±0.03) |
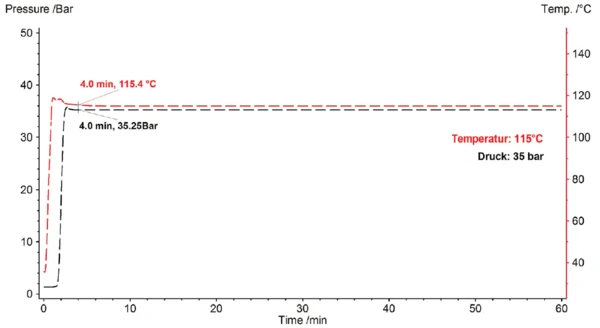
IsothermalTests at controlled and constant temperature are called isothermal.Isothermal pressureless investigation of the OxidationOxidation can describe different processes in the context of thermal analysis.oxidation behavior is usually carried out by heating the sample to the appropriate IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature under a protective gas and, after a short stabilization phase, switching the purge gas from inert to oxidizing (ISO 11357-6). In contrast with this, for investigation under increased pressure, the pressure is initially controlled for a 5-minute IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment at room temperature and is then set to the desired value (here 35 bar); next, after a stabilization phase, the temperature is increased at a constant heating rate of 10 K/min (ASTM E2009-08). Figure 2 shows the course of temperature and pressure as a function of time.
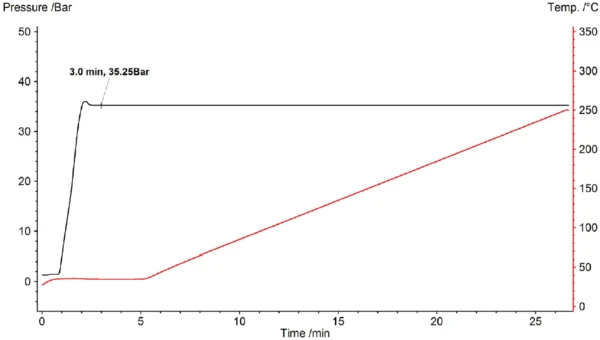
Results and Discussion
Various cooking oils were investigated at an oxygen pressure of 35 bar (gas flow 100 ml/min) by means of the linear heating rate. The OxidationOxidation can describe different processes in the context of thermal analysis.oxidation behavior results are portrayed comparatively in figure 3 for all oils.
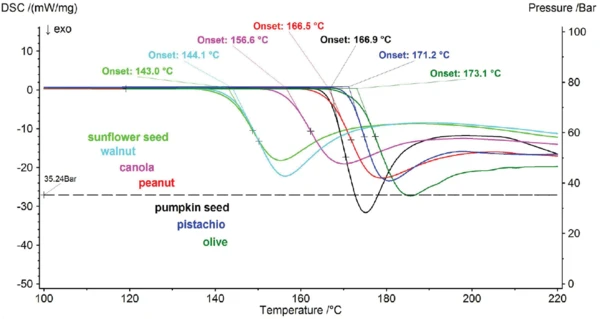
Under these measurement conditions, the sunflower seed and walnut oil exhibit the highest reactivity while the olive oil has the highest resistance to OxidationOxidation can describe different processes in the context of thermal analysis.oxidation. As a criterion for the start of the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic combustion reaction, the extrapolated onset was used. The resulting values for all oils investigated are summarized in table 3.
Table 3: OxidationOxidation can describe different processes in the context of thermal analysis.Oxidation behavior of all cooking oils in oxygen (35 bar / 100 ml/min)
Origin | Sunflower seed | Walnut | Canola | Peanut | Pumpkin seed | Pistachio | Olive |
|---|---|---|---|---|---|---|---|
| Manufacturer | 1 | 2 | 3 | 2 | 2 | 4 | 5 |
Extraoplated Onset [°C] | 143.0 | 144.1 | 156.6 | 166.5 | 166.9 | 171.2 | 173.1 |
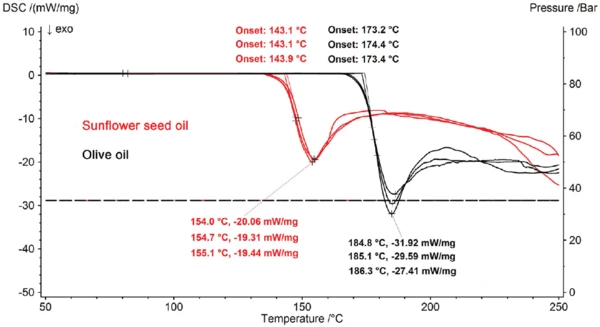
A comparison of the repeated measurements on sunflower seed and olive oil shows that there is an uncertainty of less than ± 1 K associated with determining the start of the OxidationOxidation can describe different processes in the context of thermal analysis.oxidation reaction by means of the extrapolated onset (figure 4). This provides evidence that most of the cooking oils investigated can clearly be differentiated with regard to their oxidation behavior (figure 3). The sunflower seed and walnut oils, however, have such similar values at 143.0°C and 144.1°C that a significant differentiation is not possible under these measurement conditions. For samples behaving as differently as the sunflower seed (143.0°C) and olive oils (173.1°C) (see figure 3), linear heating rate is ideal; it allows the different resistances to oxidation to be significantly differentiated. Additionally, it would be very difficult or even impossible to find a temperature with an IsothermalTests at controlled and constant temperature are called isothermal.isothermal measurement program at which both samples would react within a manageable period of time.
If the objective, however, is to differentiate between oils which exhibit very similar oxidation behavior, such as sunflower seed oil (143.0°C) and walnut oil (144.1°C) (see figure 3), then an IsothermalTests at controlled and constant temperature are called isothermal.isothermal oxidation test is advantageous. The sample is initially heated to the desired temperature at a heating rate of 100 K/min; then, after a two-minute stabilization phase, the oxygen supply valve is opened and the entire instrument is pressurized with oxygen to 35 bar (ASTM D6186-08). Figure 5 illustrates the measured temperature and pressure course. Hereby, the temperature must be selected in such a way as for the most reactive sample to be exhibiting resistance for a few minutes before the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic reaction starts.
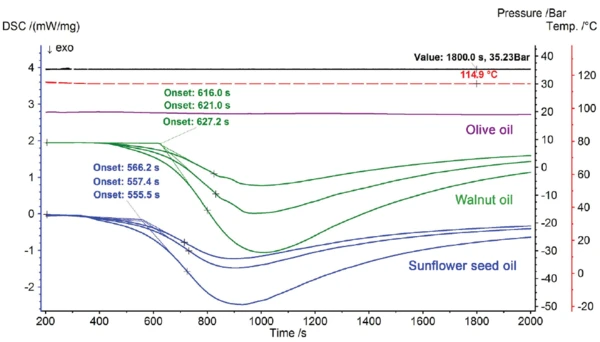
The results of the IsothermalTests at controlled and constant temperature are called isothermal.isothermal oxidation tests at 115°C and 35 bar oxygen pressure for the sunflower seed and walnut oil samples are depicted in figure 6. The start of the oxidation reaction (extrapolated onset) is defined here in seconds for better illustration. The multiple determinations for the start of oxidation show that the sunflower seed oil, at 559.7 s (± 6), has a significantly lower resistance to oxygen under these conditions than the walnut oil, at 621.4 s (± 6). At 60 s, the difference in the start of reaction is approximately ten times higher than the measurement uncertainty. The olive oil, however – also measured under these conditions for comparison – remains resistant for several hours.
Summary
The oxidation behavior of oils, greases, waxes, or polymers and hydrocarbons in general can be investigated by means of Differential Scanning Calorimetry (DSC). Various national and international standards recommend characterization using certain measurement parameters including different temperature treatments (IsothermalTests at controlled and constant temperature are called isothermal.isothermal/dynamic), crucible types (cylindrical/SFI), atmospheres (synthetic air/oxygen) or pressures (atmospheric pressure/35 bar).
For the characterization of different cooking oils, dynamic temperature control at a heating rate of 10 K/min, an oxygen pressure of 35 bar and a gas flow of 100 ml/min has proven to be an advantageous combination.
For liquid samples or for substances which change viscosity during heating, so-called SFI crucibles are especially well suited since the special shape of their bottoms prevents the sample from creeping up the crucible wall or from changing the contact area with the crucible bottom in some other way.
Samples exhibiting very similar oxidation behavior under dynamic conditions can be better characterized in certain situations by means of an IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature program. Although the appropriate IsothermalTests at controlled and constant temperature are called isothermal.isothermal temperature would first have to be determined for a series of samples, this measurement program is often more selective for similar samples.