Origin and Application
Mica schist has been mined ever since prehistoric times. Mica was actually used in window panes prior to the industrial production of glass, since its layered structure allows it to be easily broken into thin sheets. Nowadays, mica is used as a pigment in paints and cosmetics. Due to its good thermal conduction and electrical insulation properties, another important application field for mica is the electronics industry, where it is used as insulating discs for semiconductor components or as a dielectric for very low-loss capacitors [1].
Structure
Mica is the generic term for a group of minerals belonging to the sheet silicates. Their general chemical composition is DG2.3 [T4O10]X2. One layer is formed of corner connected SiO2 tetrahedrons (for T = Si); another layer consists of GO6 octahedrons. Each octahedron layer is embedded between two tetrahedron layers. This T-O-T (tetrahedral-octahedral-tetrahedral) layer sequence, however, is not neutral in charge. Charge equation is achieved by means of bridging interlayer anions (X) [2].

Measurement Conditions
- Instrument
- TG 209 F1 Libra®
- Sample
- Mica
- Sample mass
- 5.106 mg
- Crucible
- Al2O3
- Atmosphere
- Nitrogen
- Gas flow rate
- 40 ml/min
- Heating rate
- 10 K/min
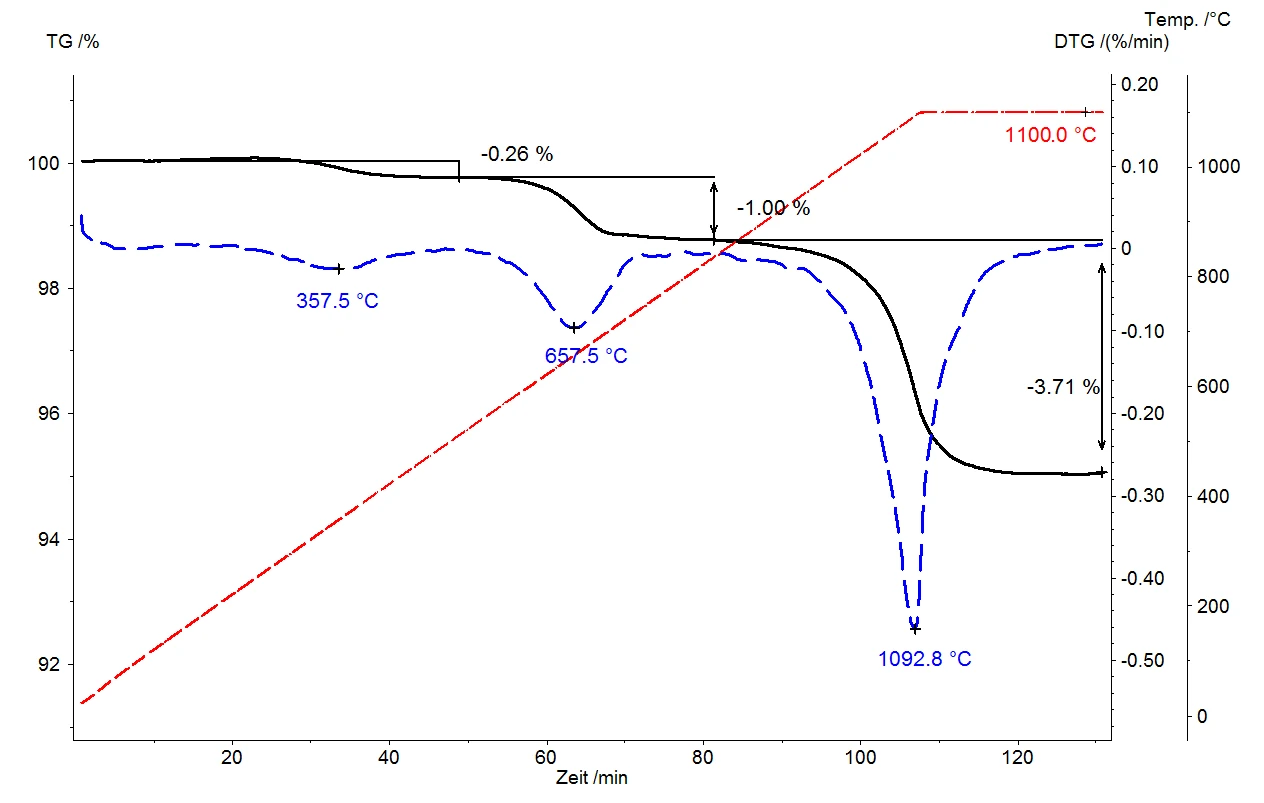
Thermogravimetry
A thermobalance can provide excellent characterization of the thermal behavior of inorganic substances. It continuously records any change in the sample composition, while the use of a dynamic heating rate (10 K/min in our example) further allows for sample occurrences to be evaluated as a function of temperature. Since the heating rate is a measure of the energy supply to the sample, it allows conclusions to be drawn regarding the required release energy and/or the binding energy of the released substances.
The example presented here with a mica sample shows three well separated mass loss steps; one at 357°C, another at 657°C and the third at 1092°C. The good separation of these mass-loss steps allows for easy quantification of the amounts of gas evolved from the samples via step evaluation at the various temperatures. The relative mass changes amount to 0.26%, 1.00% and 3.71%. The release temperature is an indicator of the strength of the adsorption or bond: the higher its value, the stronger the adsorption of the gas to the crystal surface or the bond to the crystal structure prior to release. In contrast with “flash PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis” – where the sample is heated to the maximum temperature within a few seconds and all gases are thus released very abruptly – thermogravimetry’s very variable heating rates and combination of dynamic and IsothermalTests at controlled and constant temperature are called isothermal.isothermal segments allow for the gaseous Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products to be released as a function of temperature and therefore in a stepwise fashion. Our example with mica additionally shows that a heating treatment of the sample to 1100°C at 10 K/min was not sufficient to allow for conclusion of the full Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction, and thus of course was not sufficient for its quantification either.
In fact, a subsequent IsothermalTests at controlled and constant temperature are called isothermal.isothermal phase of 30 minutes was necessary to totally complete the reaction. Such flexibility in temperature control not only facilitates quantification, but additionally allows for identification of the gases evolved from the sample during the thermogravimetric process. Well suited for this is a technique called “coupling”, where thermoanalytical measuring instruments are coupled to instruments for spectroscopic methods such as mass spectrometry or FT-IR spectroscopy.