Introduction
In pharmacy, there is hardly any active ingredient about which more has been written than acetylsalicylic acid (or ASA for short; in English-speaking countries even the brand name Aspirin™ is often used as a synonym). Its success story began at the end of the 19th century when Dr. Felix Hoffmann synthesized the substance at the BAYER laboratories for the first time without impurities. Nowadays, it is still one of the most popular pharmaceuticals used across a broad therapeutic range. It belongs to the group of non-steroidal anti-inflammatory drugs (NSAIDs) and is indicated for the treatment of pain, fever and inflammation. In addition, it is used to prevent recurrence of heart attack or stroke in high-risk patients. In 1977, ASA was added as an analgesic to the “essential drug list” of the WHO (World Health Organization). [1]
This is one of four application notes that examine in more detail the thermal behavior of acetylsalicylic acid: Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition in different gas atmospheres, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition kinetics, and the resulting gas species.
Table 1: Measurement parameters
| Parameter | Acetylsalicylic Acid |
| Sample mass | Approx. 5 mg |
| Atmosphere | Argon, nitrogen and helium |
| Crucible | Al2O3, 85 μl, open |
| Temperature program | RT to 600°C |
| Flow rate | 40 ml/min |
| Sample holder | TGA, Type P |
Experimental
The sample material, acetylsalicylic acid (CAS: 50-78-2), was acquired from Sigma Aldrich with a purity of > 99%. It is a white, crystalline powder which exists in three crystal modifications [2]. Form I, with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of about 137°C [4], is the most stable one at ambient temperature and above [3].
PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.Pyrolysis experiments are usually carried out in a nitrogen atmosphere due to its availability and comparatively low price. This is also reflected in several publications, for example [5] and [6]. To answer the question as to wheter the results obtained under nitrogen can also be generalized to other atmospheres, an experimental series was performed for studying the thermal behavior of acetylsalicylic acid as a function of the purge gas nature. Besides nitrogen, other inert gas atmospheres used were helium and argon. The corresponding measurement parameters are summarized in table 1.
For characterization of the thermal behavior, a NETZSCH TG 209 F1 instrument was employed.
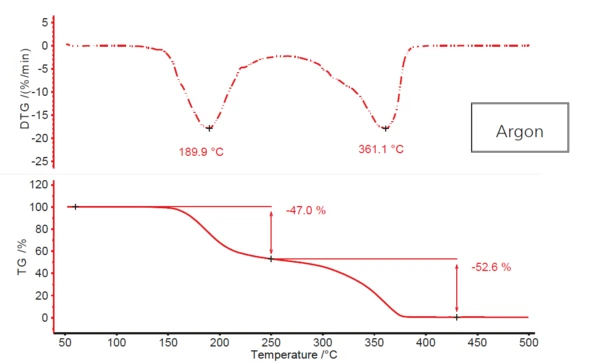
Figure 1 depicts the typical two-step Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition profile of acetylsalicylic acid, resulting from heating the substance in a flowing argon atmosphere. The first step, with a DTG peak temperature of about 190°C, exhibits a mass loss of 47%; the second step, at 361°C (again DTG peak temperature), exhibits almost 53%. However, no real plateau occurs between the two mass-loss steps. The first one merges, more or less, into the second. This indicates that maybe more than two Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition steps are involved. The possibility that a more complex mechanism such as this would be the case here is additionally supported by the fact that the second DTG peak has a clearly visible shoulder at approx. 320°C in the descending slope.
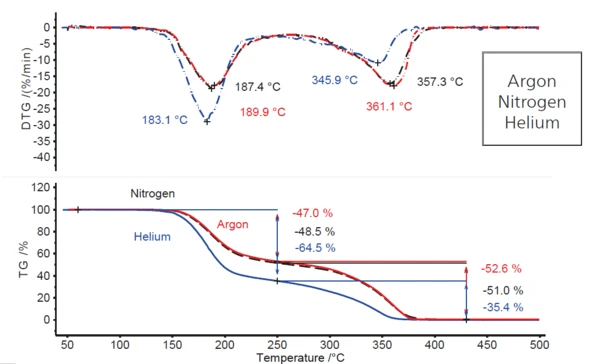
Comparing the situation in an argon atmosphere with experiments performed at identical heating rates in both nitrogen and helium atmospheres (figure 2), the behavior under nitrogen conditions is about the same as in argon, whereas it changes significantly under a flowing helium atmosphere. An increase in the first massloss step of about 18 percentage points (from 47% to almost 65%) and consequently, a decrease in the second mass-loss step of the same amount (from 53% to 35%) is observed. In addition, both mass-loss steps are shifted to somewhat lower temperatures, indicated by the decrease in the particular DTG peak temperatures (4 K to 7 K for the first DTG peak and 11 K to 15 K for the second one). This suggests that something different is going on in a helium atmosphere than in argon and nitrogen atmospheres.
In literature, a two-step mechanism with simultaneous evaporation of intermediates is proposed as the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis mechanism of acetylsalicylic acid [7]. Measurements conducted by NETZSCH in a nitrogen as well as in a helium atmosphere using hyphenated techniques, more precisely, TGA/STA in combination with FT-IR [6] and GC-MS [8], support this hypothesis. This indicates that the nature of the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products is independent of the gas atmosphere.
The major difference between all these experiments is the DensityThe mass density is defined as the ratio between mass and volume. density of the purge gas used (see table 2). It differs maximally by a factor of 10.
This suggests that a higher DensityThe mass density is defined as the ratio between mass and volume. density of the purge gas creates a higher back pressure, which results in a reduced transfer of the volatile sample components into the gas atmosphere. This effect is particularly visible when using helium, which has a much lower DensityThe mass density is defined as the ratio between mass and volume. density than nitrogen or argon. Since true Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reactions are independent of the surrounding inert gas atmosphere [10], it is perhaps the parallel evaporation that is mostly affected.
The fact that the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition takes place at slightly lower temperatures in helium (e.g., DTG peak at 183°C compared to 187°C in nitrogen and approx. 190°C in argon) is due to the higher Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of this gas (see table 3). In the temperature range where thermal radiation plays only a minor role, the sample reaches the reaction temperature in a purge gas with higher Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity somewhat earlier.
Table 2: DensityThe mass density is defined as the ratio between mass and volume. Density values at 0°C and normal pressure of various purge gases
| Gas | DensityThe mass density is defined as the ratio between mass and volume. Density / (g/cm³) [9] |
| Helium | 0.178 |
| Nitrogen | 1.251 |
| Argon | 1.784 |
Table 3: Thermal conductivity values under standard conditions of the various purge gases
| Gas | |
| Helium | 0.1567 |
| Nitrogen | 0.0260 |
| Argon | 0.0179 |
Conclusion
The present example shows that the selected gas atmosphere may have a strong impact on the thermogravimetric measurement results, even if the purge gas does not act as a reaction partner. A greatly varying gas DensityThe mass density is defined as the ratio between mass and volume. density can have an effect on the transfer of gaseous compounds from the sample surface into the surrounding gas atmosphere – especially if evaporation is involved.